Introduction
Dilated cardiomyopathy (DCMP) in Wolff-Parkinson-White (WPW) syndrome is usually considered a tachycardia-induced cardiomyopathy resulting from recurrent and/or sustained tachyarrhythmia [1]. There have been a small number of reported cases of DCMP seen in children with WPW syndrome without documented supraventricular tachycardia (SVT) [2-5]. However, adult DCMP patient with WPW syndrome not associated with inducible SVT is not well known. The relationship between accessory pathway-mediated ventricular preexcitation and left ventricular dyssynchrony-induced dysfunction has been described in patients with WPW syndrome in the absence of sustained SVT [2,6,7]. We report on catheter ablation in an adult patient with WPW syndrome for dilated cardiomyopathy in the absence of SVT.
Case
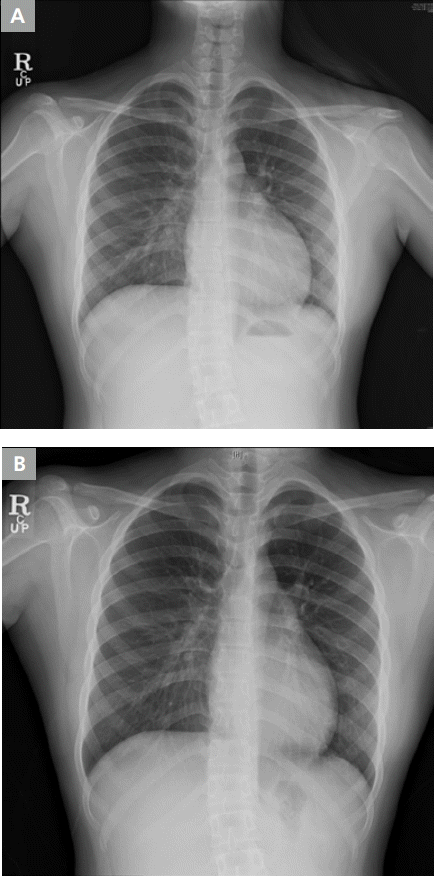
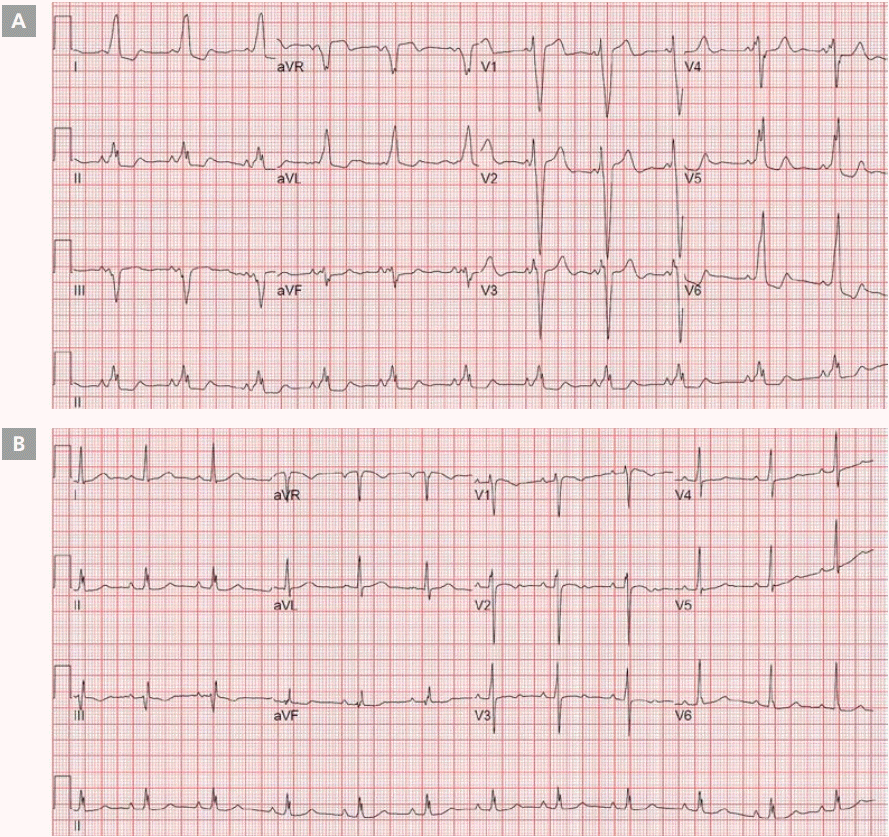
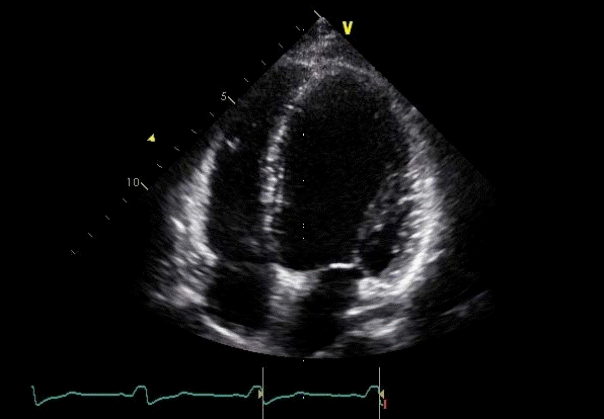
A 20-year-old female patient was diagnosed with dilated cardiomyopathy and was treated in an outpatient clinic with heart failure medication. The patient showed a decrease in the symptom of dyspnea on exertion after medication treatment. However, this symptom reoccurred over time, and ventricular function also showed deterioration on the echocardiogram. The chest radiograph is shown in Figure 1. On the electrocardiogram (ECG), a distinct delta wave pattern was observed, suggesting WPW syndrome (Figure 2A). The patient had previously demonstrated no evident symptoms such as palpitations or documented tachycardia events. The delta wave on the ECG showed an R<S pattern on V1 and positive deflection on leads I, II, and aVF; suggesting that the Kent fiber was located in the right anterolateral portion. The QRS duration was prolonged to 168 msec suggesting interventricular dyssynchrony. On the echocardiogram, the left ventricular ejection fraction (LVEF) was decreased to 30% and the left ventricle (LV) and left atrium (LA) showed enlargement (left ventricular end diastolic dimension=61 mm, left ventricular end systolic dimension=52 mm, and left atrial volume index=21.6 mL/m2, Figure 3). The radial dyssynchrony index was prolonged to 339 msec, suggestive of LV dyssynchrony.
In this patient, the possibility of myocardial dysfunction resulting from interventricular dyssynchrony due to a right anterolateral accessory pathway was raised. We carefully postulated that this explained the LV enlargement, decreased LVEF, and the progression to DCMP. Therefore, we decided to perform electrophysiology studies (EPS) and radiofrequency ablation on the accessory pathway.
The non-sedated patient presented to the EPS lab in normal sinus rhythm with a delta wave pattern. Both inguinal areas were sterilized with betadine, and both the femoral veins and the right jugular vein were punctured after instillation of 2% lidocaine.
After the femoral veins had been punctured, three 5 Fr sheaths and one 7 Fr sheath were inserted. Two quadripolar catheters were placed at high RA and RV, and a hexapolar catheter was inserted into the His bundle area. Following the right jugular vein puncture, a 7 Fr sheath was inserted with a decapolar catheter into the coronary sinus (CS). A coronary venogram was completed.
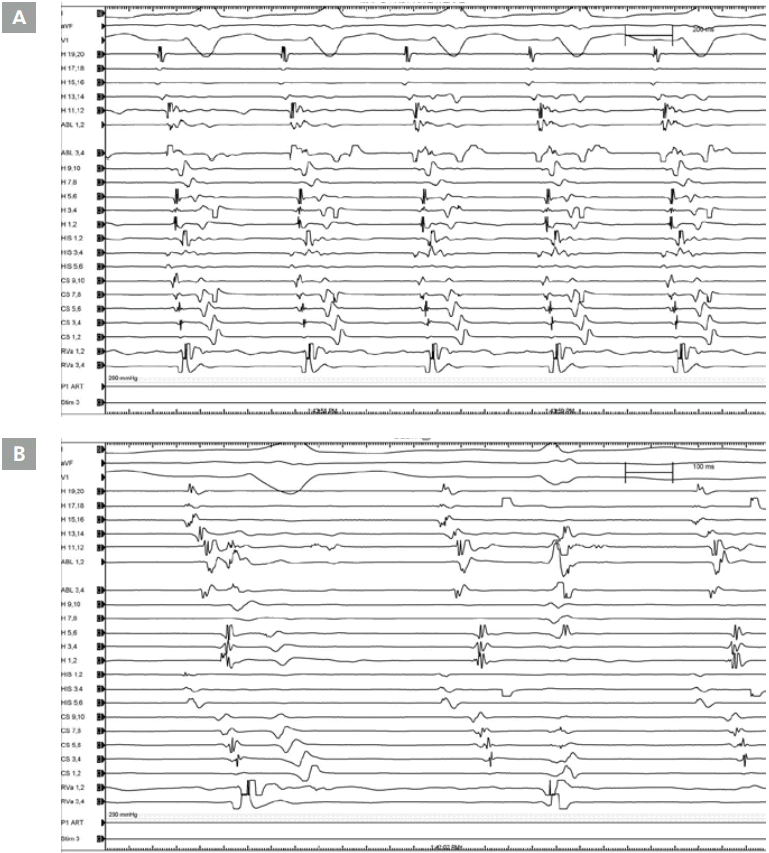
The patient’s ECG baseline showed a normal sinus rhythm with a delta wave pattern (SCL: 604 msec, AH: 27 msec, HV: 17 msec, QRS: 172 msec). During RVP, VAD was noted. After Isuprel 1 μg/min administration, VA conduction via the node was noted during RVP. Tachycardia was not induced via a programmed electrical stimulation. Mapping of the accessory pathway was performed with an EPT ablation catheter (EPT, Std, 7 Fr/4 mm tip) via the right femoral vein. A Duo-decapolar (Halo) catheter was placed at the TV annulus of the RA. During NSR, antegrade conduction of V signal was fastest at H 11, 12 (RA lateral) (Figures 4A, 5).
With visualization of the right lateral accessory pathway, a steerable ablation catheter (EPT, Std, 7 Fr/4 mm tip) was inserted into the 9 o’clock position of the TV annulus. During the second RF ablation, Kent was disappeared, but recurred. After the sixth RF ablation, the Kent was disappeared. However, after 22 minutes, antegrade conduction via the Kent was again noted. During mapping via the EPT ablation catheter, a mechanical malfunction was noted and antegrade conduction via the Kent was disappeared over a 20-minute time period. During the seventh RF ablation (1 sec), the Kent was disappeared (Figure 4B). However, following the seventh RF, AV block without antegrade conduction via the Kent after the administration of adenosine 6 mg and 12 mg intravenously. After confirming successful RF ablation of the right lateral accessory pathway, all catheters were removed, and the procedure was finished without complications.
The delta wave disappeared after radio frequency catheter ablation (RFCA) and the QRS duration shortened to 100 msec, as shown on Figure 2B.
A follow-up echocardiogram performed six months later showed a decrease in LV enlargement (left ventricular end diastolic dimension=57 mm, left ventricular end systolic dimension=44 mm), and the LVEF improved to 50%. The interventricular radial dyssynchrony index returned to a normal range of 120 msec.
After the EPS and RFCA, the patient’s symptom of dyspnea on exertion vanished. Heart failure medication was no longer needed, and the LVEF showed gradual improvement.
Discussion
Until this case, the published reports about myocardial dysfunction, or DCMP in WPW syndrome without tachycardia, usually discussed patients who presented with symptoms during early infancy. These patients started medication or EPS in early childhood [8,10]. Also, the accessory pathway was usually located in the right anterior septal area of the RA [9,11]. Our case discusses an adult WPW patient with DCMP, without tachycardia, and the accessory pathway was located in the right anterolateral wall of the RA. The evidence of dyssynchrony was evident on both the ECG as a prolonged QRS duration, and the echocardiogram as an abnormal interventricular radial dyssynchrony index. This dyssynchrony vanished after EPS and RFCA as evidenced by a shortened QRS duration and normalization of the interventricular radial dyssynchrony index. We can conclude that the accessory pathway in WPW syndrome in adults can be a cause of DCMP. We can also conclude from our case that EPS and RFCA of the accessory pathway results in improved DCMP, even in patients diagnosed in adulthood. The accessory pathway in the right septal area is known to be the cause of dyssynchrony and DCMP, as previously reported [2,5,9,11]. However, we can also conclude that the accessory pathway located at the right anterolateral area can be the cause of interventricular dyssynchrony, and can cause DCMP eventually since it is on the right side. In conclusion, WPW syndrome with a right-sided accessory pathway can be the cause of DCMP, and aggressive EPS and RFCA treatment may be necessary for this group of patients.