Introduction
The J wave is defined as an electrocardiogram (ECG) deflection with a dome or hump morphology, immediately following the QRS complex [1]. The cellular mechanism of J wave formation consists of transmural differences in the early phases of the action potential [2,3]. The augmentation of the J wave or the appearance of ST-segment elevation is caused by an outward shift in the repolarizing current owing to a decrease in sodium- or calcium-channel currents, or an increase in Ito, IK-ATP, IK-Ach, or other outward currents [1].
However, the clinical significance of the J wave has not yet been fully evaluated. Although this condition is usually considered benign, several studies have revealed a relationship between idiopathic ventricular fibrillation and J point elevation [4,5]. Moreover, it is not clear whether the J wave represents the genetic variant of early repolarization or whether it results from delayed depolarization associated with structural abnormality of the myocardium [6].
Recently, the appearance of J wave in the inferior leads has been associated with an elevated risk of fatal arrhythmia or sudden cardiac death [5,7,8]. Some researchers have suggested that J wave appearance is related to the risk of acute myocardial infarction (AMI) [9]. Based on previous reports, we hypothesized that the appearance of J waves in inferior leads following inferior-wall AMI might be associated with worse outcomes. To prove our theory, we analyzed the clinical implications of J wave before and after the revascularization of inferior-wall AMI.
We also hypothesized that reversed-J (rJ) waves in leads V1-V3 are the mirror images of J waves in the inferior leads. In AMI patients, electrocardiographic ST-segment depression is called a reciprocal change when it occurs simultaneously with ST-segment elevation in leads on the opposite side of the heart. In these patients, we hypothesized that a J wave in the inferior leads and its reciprocal change, the rJ wave in leads V1-V3, might share a similar clinical meaning.
Materials and Methods
Our study protocol was approved by the institutional review board of Seoul National University Hospital and was carried out in accordance with the Declaration of Helsinki. Patient consent was waived because it was not practical to obtain consent from large numbers of patients for a retrospective review study, and the data were analyzed anonymously.
Patients
The study population consisted of 336 consecutive patients without a previous diagnosis of coronary artery disease (CAD). From January 2005 to March 2010, they had received urgent percutaneous coronary intervention (PCI) at Seoul National University Hospital to treat inferior-wall AMI. A previous diagnosis of CAD was defined as a history of myocardial infarction (MI), coronary revascularization, or significant CAD confirmed by prior catheterization. PCI was performed ≤90 minutes of the patient’s visit to the hospital for ST-elevation myocardial infarction (STEMI), and ≤48 hours for non-ST-elevation myocardial infarction (NSTEMI). Patients were included in the study if they were followed up over a 6-month period and received adequate medical support throughout, including dual antiplatelet agents (aspirin and clopidogrel). We excluded patients with bundle-branch block (n=9) and those who had cardiac pacemakers at baseline (n=1). We also excluded patients whose follow-up duration was less than 6 months (n=23) and one patient who died during PCI (n=1). A total of 302 patients satisfied the inclusion criteria.
ECG Analysis
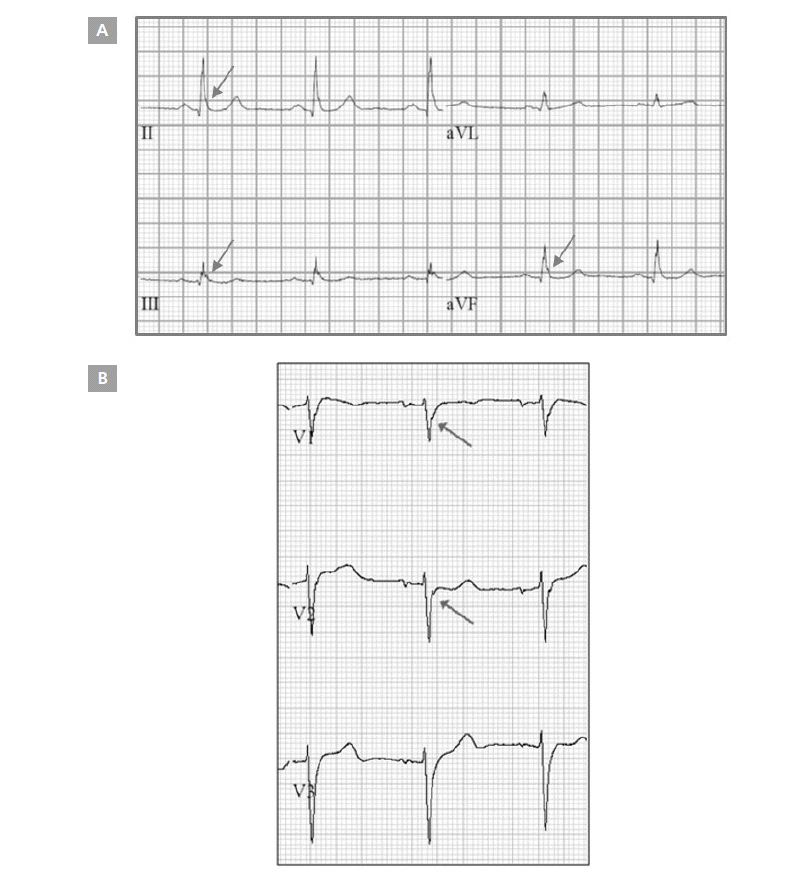
Patients’ ECGs, obtained 1 day after PCI, were retrospectively analyzed by 2 independent cardiologists using a previously described method. The J wave was defined as either a slurred or notched J point elevation in at least 2 consecutive leads [10]. The J point amplitude was measured at the QRS-ST junction in the case of slurred J waves, or at the peak J point in the case of notched J waves. J point amplitude was measured relative to QRS onset in order to minimize any baseline wandering effect, as previously described (Figure 1) [11].
The J group consisted of patients whose J waves were >0.1 mV in ≥2 leads (among leads II, III, and aVF) and/or who had 2 rJ waves that were >0.1 mV in ≥2 leads among leads V1, V2, and V3. Patients were grouped into the big-J group (a subgroup of the J group) if the amplitude of ≥2 of their J waves or ≥2 of their rJ waves was >2 mV. The non-J group consisted of patients who had neither J nor rJ waves in any of the 12 leads.
Next, pre-PCI ECGs obtained from patients ≤24 hours before PCI were reviewed in order to identify the J wave in the same manner.
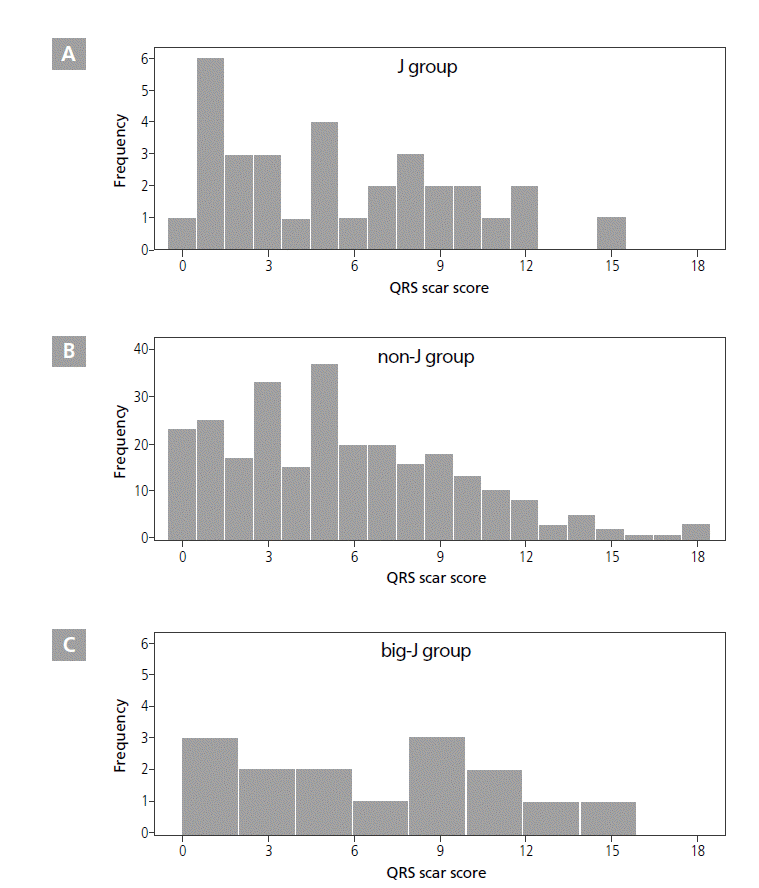
The QRS scar score taken 1 day after PCI was used for estimating left ventricular (LV) scar amount in each patient, calculated according to instructions from previous studies [12,13]. This score considered Q and R wave duration, R/Q and R/S amplitude ratios, R and S wave amplitudes, and R wave notches. The score had 32 total possible points, each of which represented 3% of the LV mass. expected to accentuate the early repolarization pattern by inhibiting the sodium channel current. The J wave could be the genetic variant of early repolarization, or it could be caused by delayed depolarization associated with a myocardial disorder. However, in this study, the distinguishment is still remained questionable, and this could be one of the important limitations of our investigation. Thus, further well-designed prospective studies are needed.
To exclude the possibility that MI-related scar was a prognostic indicator for patient mortality [18], we compared the QRS scar score of the J group with that of the non-J group. QRS scar scores quantify cardiac scarring in ischemic and non-ischemic cardiomyopathy patients [12,19], and their results have been positively associated with mortality [13,20]. In this study, no significant difference was found between the scar scores of the 2 groups (5.53 vs. 5.61, respectively, p=0.916). This means that the prognostic power of J waves and rJ waves was not affected by scar size. In fact, the relationship between scar size and magnitude of injury currents is not well defined, and no definitive methods are available for quantifying injury currents.
A recent study found that early repolarization before successful reperfusion after AMI increased the risk of VF ≤48 hours after event onset [10]. Our study showed similar results-i.e., the pre-PCI J wave was associated with post-PCI ventricular arrhythmic events (p=0.016, HR=31.671, CI 1.915-523.852), but not with mortality. These results imply that pre-PCI J point elevation is a leading cause of ventricular arrhythmia ≤48 hours after PCI.
In previous studies, many clinical factors, such as old age, prior MI, congestive heart failure, and stroke, were associated with poor outcomes in AMI patients [21]. We attempted to control and adjust for such known predictors during our statistical analyses. However, it is impossible to consider all confounding variables because many unknown and random factors can affect the prognosis of AMI patients. In our analyses, we adjusted for age, sex, hypertension, diabetes dyslipidemia, LVEF <35%, CKD, number of involved vessels, STEMI/NSTEMI, and ST-segment changes in the precordial leads as confounding factors. None of these factors, except for LVEF, directly reflected AMI-induced cardiac conditions. Therefore, clinicians have used simple risk-scoring methods, such as the Thrombolysis in Myocardial Infarction (TIMI) system, to discern the prognosis of AMI patients [22,23]. However, this system cannot be used as the sole means of determining patient disposition [24].
If it were possible to estimate residual injury currents based on J and rJ waves from the ECGs of AMI patients, then J and rJ waves could be objective markers of current cardiac status. This system would be useful for evaluating AMI patients after successful revascularization. It is known that precordial ST-segment depression upon admission during inferior-wall AMI predicts higher hospital mortality rate and suggests worse long-term prognosis after discharge [25]. Thus, rJ wave evaluation could better help predict AMI patient prognosis as compared to the use of J waves alone. Hence, combining J wave with rJ wave assessment might be a more powerful tool for predicting cardiac death.
The cutoff value of J waves has not been established. A previous study suggested that J point elevation >0.2 mV increased the relative risk of cardiac death to 2.98 [26]. In our study, high-amplitude J waves in the inferior leads and/or rJ waves were possible prognostic factors for all-cause and cardiac mortality in inferior-wall AMI patients.
Study Limitations
When developing any useful predictive marker, accuracy and simplified criteria for general clinical use should be considered. This study showed the power of J waves and rJ waves for predicting mortality in inferior-wall AMI patients. However, their reverse-predictive value could be low, because J wave or rJ wave detection can be confounded by factors such as ST-segment elevation or bundle-branch block, and only trained ECG interpreters can discriminate these waves accurately. Therefore, J waves and rJ waves are limited as sole independent predictors of mortality after AMI.
Another limitation of this study was the size of our subject population. The total of 302 patients might be too small to definitively establish J waves and rJ waves as predictors of mortality after AMI. Therefore, a larger-scale, long-term follow-up study is needed to clarify the prognostic power of J waves and rJ waves in inferior-wall AMI patients.
Lastly, among 29 deceased patients, 10 died of non-cardiac causes ≤6 months of AMI occurrence. Although they were significantly older than patients who survived or died from cardiac
Definition of Variables
Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, and/or the use of antihypertensive medication. Diabetes was defined as either fasting blood sugar level >126 mg/dL, a 2-hour blood sugar level >200 mg/dL, or the use of blood glucose-reducing medications. The NCEP ATP-III definition was used to ascertain the presence of dyslipidemia [14]. Transthoracic echocardiography for measuring left ventricular ejection fraction (LVEF) was performed ≤1 day after PCI and confirmed by 2 expert cardiologists. A stroke was defined as the rapid loss of brain function due to the disturbance in blood supply; stroke was classified as ischemic if a brain CT or MRI confirmed no evidence of hemorrhage. Chronic kidney disease (CKD) was defined as the presence of kidney damage or glomerular filtration rate (MDRD-estimated GFR <60 mL/min/1.73 m2 ) for >3 months. Heart rate was analyzed based on pre- and post-PCI recordings.
Study Design
This retrospective review study obtained mortality data from the National Statistical Office in Korea. Each case was assessed as either cardiac death or non-cardiac death. The study’s primary endpoint was cardiac death ≤6 months after AMI, and the secondary endpoints were all-cause mortality ≤6 months and fatal and non-fatal ventricular arrhythmic events (including sustained ventricular tachycardia [VT] or ventricular fibrillation [VF]) ≤48 hours after PCI. Mortality data and QRS scar scores were compared among groups, and the power of the rJ wave for predicting death was investigated.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation. The Student t-test was used for data comparison. Categorical variables were compared using Fisher’s exact test and the χ2 test. A survival analysis that included cardiac death and all-cause mortality was conducted among subjects using a Cox regression analysis. Statistical analyses adjusted for age, sex, hypertension, diabetes, dyslipidemia, LVEF <35%, CKD, number of involved vessels, STEMI/NSTEMI, and ST-segment changes in precordial leads. P values <0.05 were considered statistically significant. Analyses were performed using IBM SPSS Statistics software version 19 (IBM Corp., Armonk, NY, USA).
Results
Demographic features of enrolled patients
The clinical and demographic features of the J group, big-J group, and non-J group are shown in Table 1. Among 302 analyzed patients, 26 showed post-PCI J waves in the inferior leads, 2 showed rJ waves only in leads V1-V3, and 4 had both inferior J and rJ waves in leads V1-V3. These 32 patients were classified into the J group. The big-J subgroup consisted of 14 patients. The remaining 270 patients were included in the non-J group.
Thirty patients had pre-PCI J waves, and 13 patients (43.3%) had high-amplitude pre-PCI J waves. Among 30 patients, 16 patients (53.3%) did not show J waves after revascularization. Newly developed post-PCI J waves were found in 18 patients.
All-cause Mortality and Cardiac Death
Among the total 302 patients studied, 29 (9.6%) died ≤6 months of AMI. The causes of death were as follows: 19 due to cardiac causes, 2 due to severe pneumonia, 2 due to stomach and bladder cancer, 1 due to cerebral stroke, and 5 due to unknown causes. Of the total 29 deceased patients, 9 (28.1%) were in the J group and 20 (7.4%) were in the non-J group. In the big-J group, 5 patients (35.7%) died. Of all the patients studied, 19 (6.3%) died from cardiac causes-5 (15.6%) in the J group and 14 (5.2%) in the non-J group. Among the big-J group patients, 4 (28.6%) died from cardiac causes.
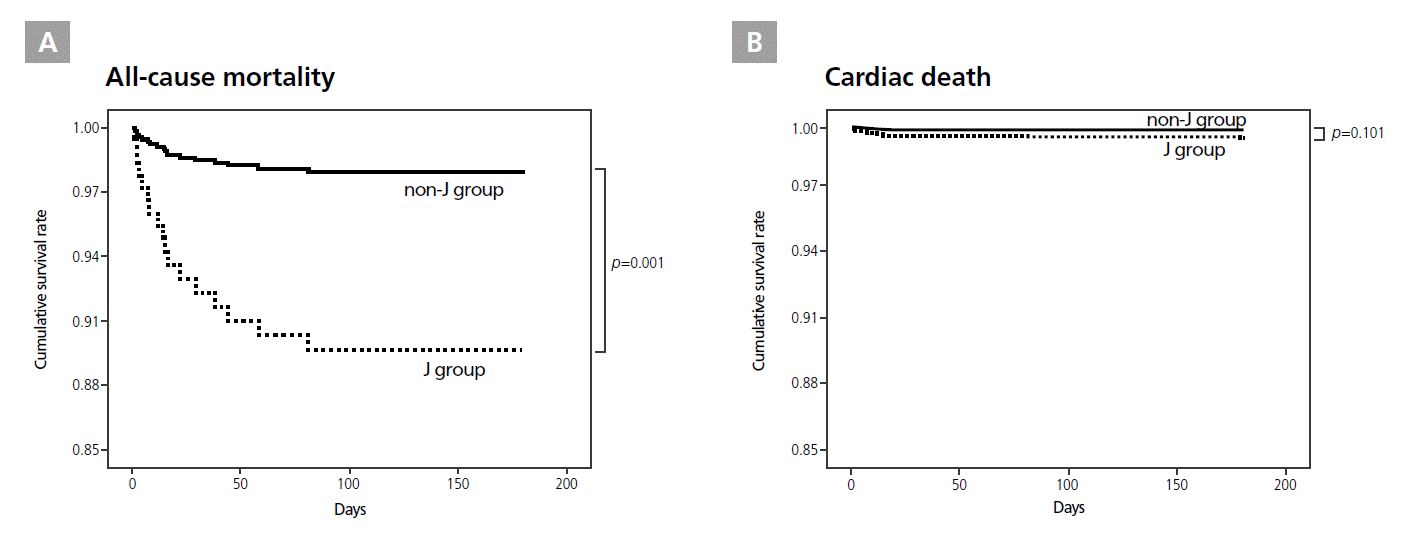
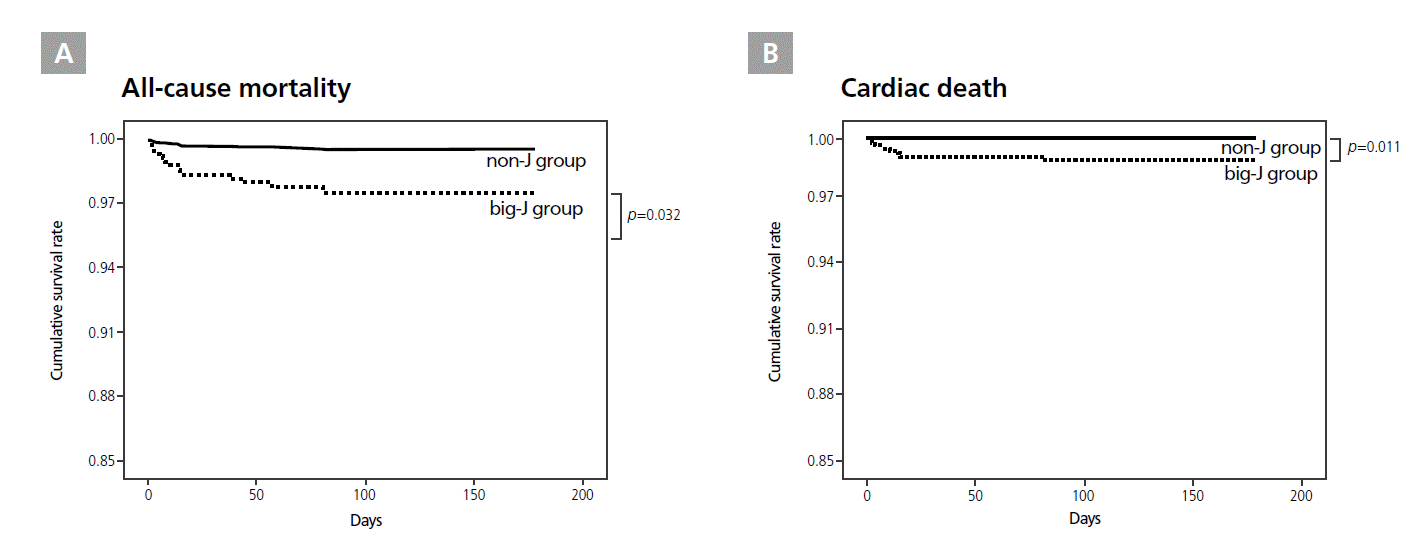
The J group had a significantly higher all-cause mortality than the non-J group (p=0.001, HR=5.376, CI 1.904-15.181, Figure 2A). However, no difference was found in cardiac deaths between the 2 groups (p=0.101, Figure 2B). The big-J group had significantly higher rates of all-cause mortality (p=0.032, HR=5.768, CI 1.165-28.561, Figure 3A) and cardiac mortality (p=0.011, HR=32.712, CI 2.203-485.723, Figure 3B) than the non-J group. All-cause or cardiac mortality did not differ between patients with or without pre-PCI J waves, regardless of their amplitude.
Ventricular Arrhythmic Events
Among the total 302 patients, 9 ventricular arrhythmic events (4 VT, 2 VT/VF, and 3 VF) occurred, including 5 fatal and 4 non-fatal events of VF/VT ≤48 hours after PCI. Although the post-PCI J wave group (p=0.314) and the big-J group (p=0.361) demonstrated no significant relationship with post-PCI ventricular tachyarrhythmia, pre-PCI J waves (p=0.016, HR=31.671, CI 1.915-523.852) were significantly associated with post-PCI ventricular arrhythmia.
Prognostic Power of rJ Waves in Leads V1-V3
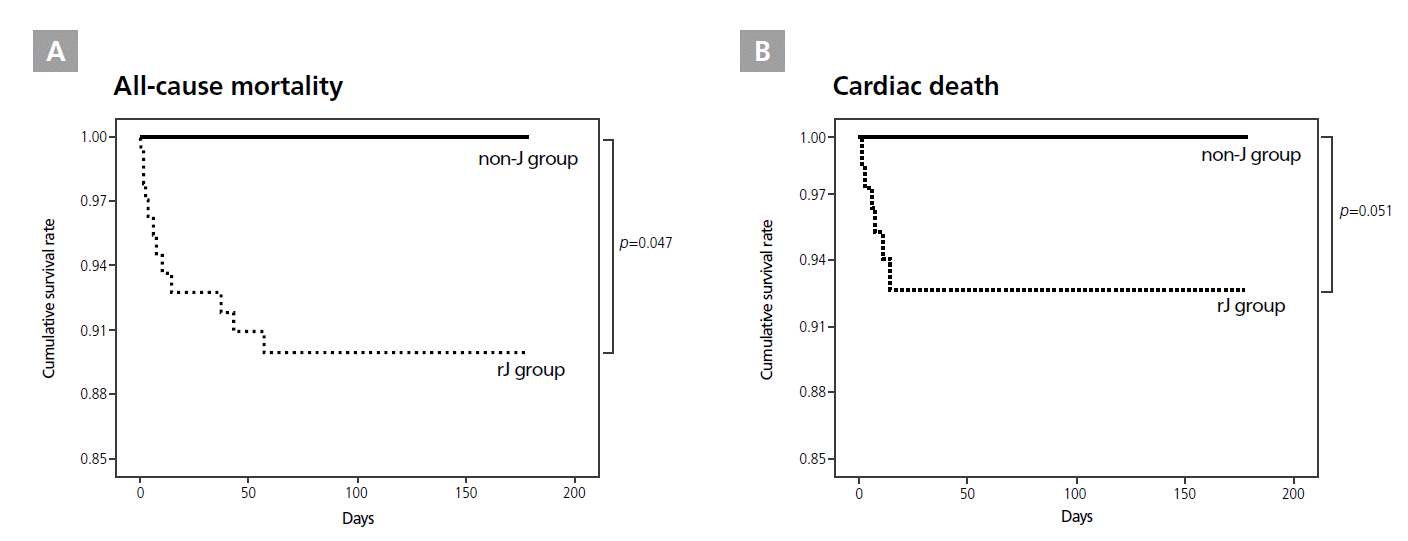
All-cause mortality was significantly higher in patients with post-PCI rJ waves (n=6) in leads V1-V3 than in the non-J group (p=0.047, HR=13.874, CI 1.035-186.021, Figure 4A). However, cardiac deaths had only marginal significance in the post-PCI rJ group compared with the non-J group (p=0.051, HR=16.636, CI 0.992-279.075, Figure 4B). All-cause or cardiac mortality did not differ between patients with or without pre-PCI rJ waves.
QRS Scar Score
The QRS scar score of the J group was 5.53±4.03, and the range was 0-15. The score of the non-J group was 5.61±4.02, and the range was 0-18. No significant difference was seen between these 2 groups (p=0.916). The QRS scar score of the big-J group was 6.64±4.57, and the range was 0-18. The QRS scar score in the big-J group also did not significantly differ from that in the non-J group (p=0.395). The QRS scar score distribution for each group is shown in Figure 5. The presence of pre-PCI J waves was not associated with the QRS scar score (4.67±4.26 with pre-PCI J waves vs. 5.71±4.26 without pre-PCI J waves, p=0.211). In addition, the QRS scar scores did not differ between deceased and living patients, regardless of the cause of death (all-cause deaths, 6.31±4.65; deaths from cardiac causes, 7.47±4.74; living patients, 5.53±3.95; all-cause deaths vs. living patients, p=0.389; deaths from cardiac causes vs. living patients, p=0.096).
Discussion
In this study, we found that patients in the J group had increased all-cause and cardiac mortality, and that rJ wave (as a reciprocal change of the J wave in the inferior leads) could be a prognostic factor of all-cause and cardiac mortality in patients with inferior-wall AMI. The pre-PCI J wave was associated with ventricular tachyarrhythmias after PCI.
Because J waves can appear in ischemic conditions, researchers in previous studies hypothesized that J waves would occur in patients with MI [9]. Moreover, some researchers thought that the J point could be elevated as a result of injury currents during AMI and pericarditis [15]. Yan et al. [16] reported that acute regional infarction resulted in heterogeneous loss of Ito-mediated epicardial action-potential domes across the ischemic border; this would lead to phase 2 reentry and cause R-on-T extrasystole that could result in VF. However, no previous studies have examined the power of J point elevation for predicting mortality after MI. In this study, the J wave and its reciprocal change (rJ wave), observed 1 day after revascularization therapy in inferior-wall AMI patients, were considered to have resulted from ischemia-induced injury currents. Thus, these events could be considered markers of poor cardiac condition and increased mortality.
It is unresolved whether J point elevation 1 day after PCI is in fact an Ito-mediated J wave or whether it represents delayed activation. The morphological definition of the terminal QRS abnormality following AMI is sometimes confusing [15]. Antzelevitch et al. [17] recommended that if the potential is delayed activation, heart rate acceleration or quinidine use would be causes (age: 80.5±10.2 years vs. 65.0±11.4 years, respectively), the large number of deaths from non-cardiac causes and 5 from unknown causes could have led to selection bias in the statistical analyses.
J waves in inferior leads and their reciprocal changes, rJ waves in the right precordial leads, could be used together as a novel prognostic marker of mortality in successfully revascularized patients with inferior-wall AMI, independent of cardiac scar size. Even so, pre-PCI J waves were significantly associated with a higher incidence of post-AMI ventricular arrhythmia.