Introduction
An electrophysiology study allows us to understand and identify effective therapy for patients with various arrhythmias. Radiofrequency (RF) catheter ablation is very effective for supraventricular tachycardia in patients with an accessory pathway [1-3]. An intracardiac electrogram (ECG) using a multiple electrode catheter in the coronary sinus provides useful diagnostic information and mapping data for patients with left-sided bypass tract-mediated tachycardia [4,5]. However, these recordings occasionally incorrectly localize a left-sided bypass tract. The most common cause of ablation failure is incorrect localization of the accessory pathway. Because accessory pathways usually have an oblique course to the atrioventricular groove [6-8], it is occasionally difficult to localize the bypass tract. Direct intracardiac ECGs of the mitral annulus (MA) more precisely localize left-sided bypass tract ablation sites compared with intracardiac ECGs of the coronary sinus (CS) [9]. Several anatomical studies using computed tomography (CT) or cadaveric heart specimens have reported that the distance between the CS and MA varies significantly [9-13]. However, electrophysiologists depend on fluoroscopy to perform RF catheter ablation in patients with a left-sided bypass tract. The purpose of this study was to evaluate the anatomical relationship between the CS and MA in living bodies by using fluoroscopy.
Subjects and Methods
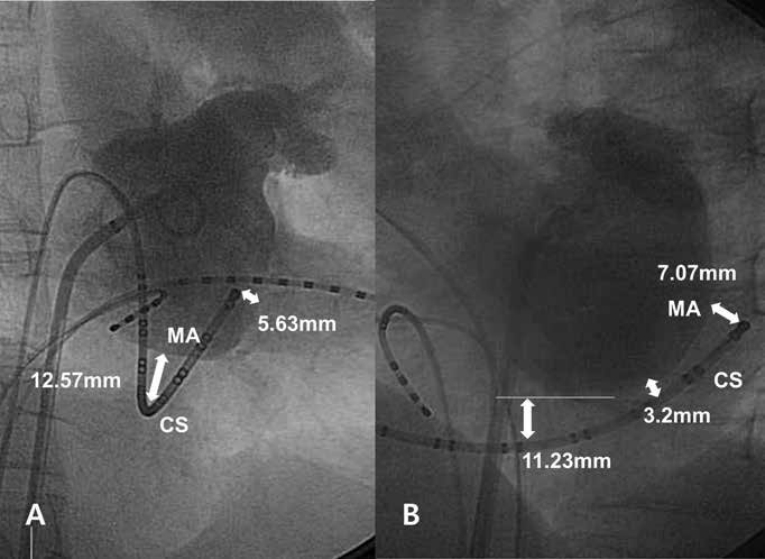
We prospectively enrolled 43 patients who underwent RF ablation for 42 left-sided accessory pathways and one paroxysmal atrial fibrillation from October 2008 to December 2010. A left atriogram was performed using a pigtail catheter via the transseptal approach after injecting 30 ml of undiluted non-iodinated contrast material during two or more cardiac cycles. The distances between the CS and MA were measured at 30° right anterior oblique (RAO) and 60° left anterior oblique (LAO) projections at the end of ventricular systole and diastole at the proximal, middle, and distal levels of the CS. The measurements were calibrated using the interelectrode spacing of a multiple electrode catheter. The position of the CS was determined using a 20 electrode steerable catheter (Duo-decapolar catheter, St. Jude Medical, Inc. Minnetonka, MN, USA) inserted into the CS under fluoroscopic guidance. The location of the CS was compared between the LAO and RAO projections using the position of each electrode of the CS catheter. The distance between the CS and MA at the distal CS level was defined as the distance between the distal tip of the Duo-decapolar catheter and intersection point of a perpendicular line from the catheter and MA under fluoroscopic-guidance (Figure 1). The distance between the CS and MA at the proximal CS level was defined as the distance between the lowest line parallel to the MA and the catheter point located at the ostium of the CS by visually inspecting the LAO projection (Figure 1B). The mid-CS level was defined as the level between the proximal CS (6 o’clock) and the distal CS (3 o’clock). This study protocol was reviewed and approved by our institutional review board, and written informed consent was obtained from the guardians of each participant.y
Results
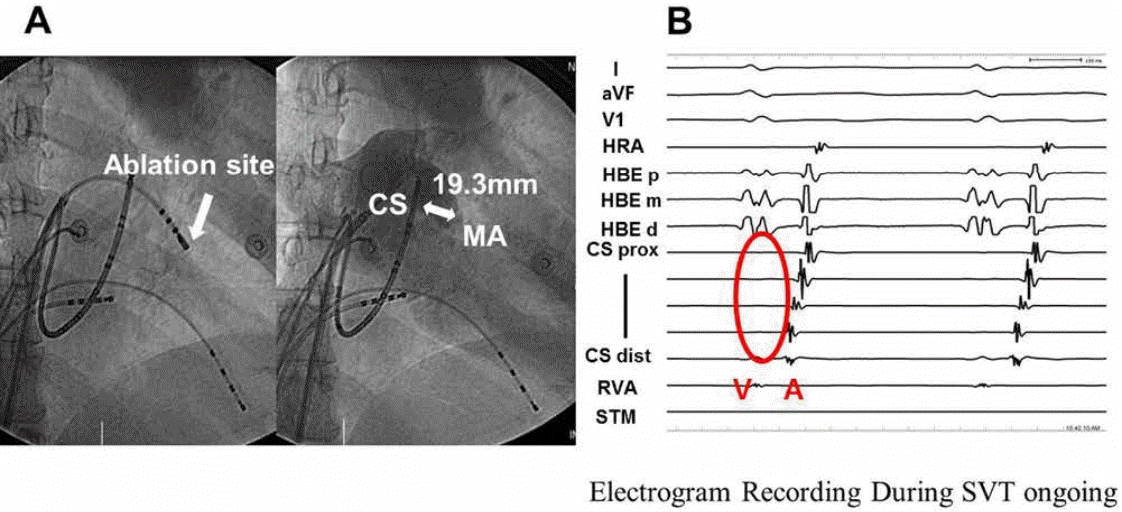
A total of 43 patients (age, 43±15 years; 29 men) were analyzed. None of the patients had a history of coronary artery disease or heart failure. All patients had normal systolic function (left ventricular ejection fraction: 64±1%). The patient baseline characteristics are shown in Table 1. The distances between the CS and MA at the RAO projection were 9.74±3.50, 3.86±2.58, and 9.02±6.04 mm during systole and 12.89±5.59, 3.97±3.24, and 10.71±4.12 mm during diastole at the proximal, middle, and distal CS, respectively. The distances between the CS and MA at the LAO projection were 6.84±2.77, 1.80±1.51, and 4.57±3.24 mm during systole and 9.91±3.25, 4.21±3.59, and 7.02±3.12 mm during diastole at the proximal, middle, and distal CS, respectively (Table 2). The mean distance between the CS and the MA was 7.04 mm in all positions (range, 0–33.36 mm). No ventricular electrical activation was seen on the intracardiac ECG of the CS during ongoing supraventricular tachycardia in one case (Figure 2), which was the result of extreme abnormal anatomical discrepancy between the CS and MA. The differences between the distance at the RAO projection and distance at the LAO projection were 2.9±3.8, 2.1±3.0, and 4.5±6.5 mm during systole and 3.0±4.9, 0.2±5.1, and 3.7±5.4 mm during diastole at the proximal, middle, and distal CS, respectively. Significant differences in the distance between the CS and MA were observed according to the projections, except for the distance during diastole at the middle CS (Table 3).
Discussion
The anatomical relationship between the CS and MA in living bodies was evaluated using fluoroscopy. Several studies have evaluated the relationship between the CS and MA by using multi-slice CT on cadaveric human hearts [9-12]. Our results provide direct anatomical insight during catheter ablation in patients with a left-sided bypass tract because RF catheter ablation procedures depend on fluoroscopy. Shinbane et al. [9] demonstrated the anatomical relationship between the CS and MA in 10 normal adult cadaver hearts. Mean distances between the CS and MA were 14.1±3.1, 10.2±4.9, and 10.7±3.5 mm at 20, 40, and 60 mm, respectively, from the CS ostium. Another study conducted with 61 excised cadeveric human hearts showed that the maximum distance from the CS to the MA was up to 19 mm (mean, 9.7±3.2 mm) [4]. Choure et al. [10] studied 25 normal individuals and 11 patients with severe mitral regurgitation (MR). They found significant variability in the relationship between the CS and MA in humans. Separation of the CS and MA increased in a lateral location and decreased in a posterior location in patients with MR. Tops et al. [11] reported that the CS is located more superior to the MA in the majority of the patients for whom multi-slice CT was used. The minimum distance between the CS and MA was 5.1±2.9 mm, which was significantly greater in patients with severe MR. Holman et al. performed a quantitative morphometric analysis of surgical anatomy in patients with Wolff-Parkinson-White syndrome. In that study, the mean distance between the CS and MA was 8±3 mm [14].
The results of our study show various anatomical discrepancies between the CS and MA. The mean distance between the CS and MA was 7.04 mm in all positions. In contrast to a previous CT study, the CS was located more inferior to the MA on fluoroscopy in most patients (Figure 1). It is helpful to determine the location of the MA from the CS catheter by using fluoroscopy in routine practice. The distances between the CS and MA at the proximal CS and distal CS were significantly greater than the distance at the middle CS (Table 2). The distance between the CS and MA was greater at the RAO projection than that at the LAO projection, except the distance during diastole at the middle CS (Table 3).
Limitations
It was occasionally difficult to fully insert the CS catheter into the CS. In all included cases, the CS catheter should be at least inserted into the CS at 3 o’clock (distal CS) in the LAO view. The CS levels were categorized by visual inspection. Therefore, it is possible that some anatomical discrepancies occurred because of individual differences in cardiac anatomy. Patients with poor left atriogram image quality were excluded. In our study, measurements were obtained between the mitral annulus displayed by left atriograms and the catheter inserted into the CS. Therefore, for a more accurate measurement, a CS venogram should have been used to identify the limits of the CS. We considered the border of the left atriogram as a mitral annulus, but there might be some differences between them.
Conclusion
Using a CS electrogram to localize the left-sided bypass tract was usually acceptable. However, various anatomical discrepancies were detected between the CS and MA. Therefore, intracardiac ECGs of the CS cannot be used to exactly localize the left-sided bypass tract. In particular, the CS was located more inferior to the MA on fluoroscopy in most patents. A precise understanding of the anatomical relationship between the CS and MA on fluoroscopy is needed to diagnose and treat certain arrhythmias.