|
|
International Journal of Arrhythmia 2013;14(2): 35-37.
|
Introduction
Amiodarone is very frequently used as an
antiarrhythmic agent owing to its wide indication
and various antiarrhythmic mechanisms. However,
physicians should be extremely cautious of the
toxicities associated with it, such as thyroid or
pulmonary changes. Although most toxic reactions
are reversible, they develop very quickly and
aggressively, and therefore, their prompt detection and management is mandatory. Here, we report a
case of amiodarone-induced pulmonary toxicity
and briefly review amiodarone-related toxicities.
Case
A 76-year-old woman visited our hospital for
dyspnea 2 years ago for the first time. She had
hypertension on medication and mild chronic
obstructive pulmonary disease (COPD). Echocardiography
revealed normal left ventricular
systolic function without any valvular diseases. A
chest computed tomography (CT) scan revealed a
large thrombus in both pulmonary arteries.
Anticoagulation with warfarin was initiated for the
treatment of acute pulmonary thromboembolism(PTE). Although PTE improved with anticoagulation
therapy, the patient was admitted to the
pulmonology department several times because of
exacerbation of the COPD. During the management
of COPD, the patient developed sustained
ventricular tachycardia (VT) without hemodynamic
collapse, and therefore, amiodarone treatment was
initiated. Following treatment with amiodarone (200 mg,
twice a day), no VT was recorded on the electrocardiogram.
However, 6 weeks later, she visited the
outpatient clinic before her appointment because of
severe dyspnea. There were crackles in both lung
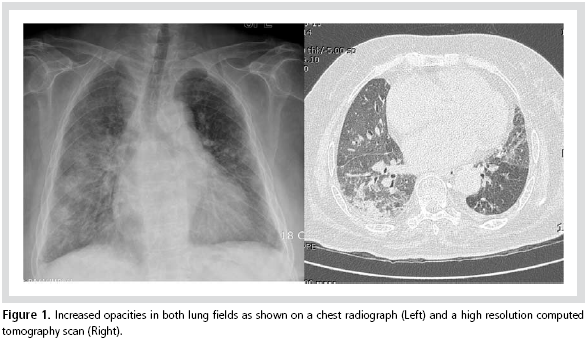
fields and the posterior-anterior view of the chest
and high resolution CT (HRCT) showed increased
opacities in both lungs (Figure 1). Arterial blood
gas analysis showed hypoxemia. Thereafter,
amiodarone treatment was discontinued and steroid
therapy was initiated. Several days later, the
oxygen requirement decreased rapidly and the
haziness in both lung fields also improved. After 3
months, the haziness in the lung fields disappeared
completely.
Discussion
Amiodarone is very widely used for the treatment
of atrial and ventricular tachyarrhythmia. It has
multiple effects on electrical myocardial activation,
primarily by blocking potassium, sodium, and
calcium channels, as well as the adrenergic
receptors. It can also be used to control atrial
fibrillation in patients with heart failure.
However, several toxicities associated with
amiodarone have also been widely reported.
Pulmonary toxicity, a major cause of death, is an
amiodarone-related toxicity.1 Thyroid dysfunction
has been reported in up to 20% of the cases in
which high doses of amiodarone were administered
and approximately 3-4% of the cases in which low
doses of amiodarone were administered.2 Cardiac
toxicities, such as sinus bradycardia and QT
prolongation; hepatotoxicity; ocular changes, such
as corneal microdeposits and optic neuropathy; and
skin reactions are other common complications that
are noted in clinical settings.
The incidence of pulmonary toxicity is
approximately 5%.3 The onset time often ranges
from several months to several years after
initiation of amiodarone treatment, and varies
among cases.2,4 The cumulative dose rather than the
serum level is major determinant of toxicity.5 It is
known that a maintenance dose of <305 mg/day
does not cause pulmonary toxicity.6 However, our
patient was on a maintenance dose as low as 200
mg/day.
Pulmonary manifestations of amiodaroneinduced
pulmonary toxicity include chronic
interstitial pneumonitis, organizing pneumonia,
acute respiratory distress syndrome, and even a
solitary pulmonary mass-like lesion of fibrosis.
Chronic interstitial pneumonitis is the most
common pulmonary manifestation. It clinically
presents as nonproductive cough and dyspnea.
Weight loss and fever can also be observed in
certain cases. Pulmonary toxicity is more common
in elderly patients, patients with preexisting lung
diseases, and those receiving amiodarone treatment
for 6 to 12 months.1 The other risk factors include
history of cardiothoracic surgery, the use of high
oxygen mixture, and co-existing respiratory
infection.
In the present case, posterior-anterior view of
the chest and pulmonary HRCT revealed diffuse or
localized interstitial or alveolar opacities. Although
bronchoalveolar lavage and lung biopsy could be
helpful in such cases, their results are variable
without pathognomonic findings.
Amiodarone-induced pulmonary toxicity is rarely
fatal and mostly reversible. Discontinuation of
amiodarone treatment is the first-line treatment. Because of the fatty accumulation and long halflife
of amiodarone, pulmonary manifestation can
persist even after discontinuation of amiodarone
treatment.
Therefore, glucocorticoid treatment is commonly
used for symptomatic amiodarone-induced
pulmonary toxicity. The common glucocorticoid
dose at treatment initiation is 40 to 60 mg of
prednisone per day. It can be tapered over a period
of 2 to 6 months. As described above, because of the
prolonged systemic effect of amiodarone,
pulmonary symptoms can recur after steroid
withdrawal. Glucocorticoid treatment at a reduced
dose can be continued to control the symptoms.
Amiodarone should be avoided in patients with a
previous history of amiodarone-induced pulmonary
toxicities.
References
- Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced
pulmonary toxicity.
Br J Clin Pharmacol.
2008;66:82-87.
- Vorperian VR, Havighurst TC, Miller S, January CT. Adverse
effects of low dose amiodarone: a meta-analysis.
J Am Coll
Cardiol.
1997;30(3):791-798.
- Amiodarone Trials Meta-Analysis Investigators. Effect of
prophylactic amiodarone on mortality after acute myocardial
infarction and in congestive heart failure: meta-analysis of
individual data from 6500 patients in randomised trials.
Lancet.
1997;350:1417-1424.
- Kay GN, Epstein AE, Kirklin JK, Diethelm AG, Graybar G, Plumb
VJ. Fatal postoperative amiodarone pulmonary toxicity.
Am J
Cardiol
1988;62:490-492.
- Ott MC, Khoor A, Leventhal JP, Paterick TE, Burger CD.
Pulmonary toxicity in patients receiving low-dose amiodarone.
Chest.
2003;123:646-651.
- Dusman RE, Stanton MS, Miles WM, Klein LS, Zipes DP, Fineberg
NS, Heger JJ. Clinical features of amiodarone-induced pulmonary
toxicity.
Circulation.
1990;82(1):51-59.
|
|
|