|

Introduction
Anatomic substrates of normal or abnormal
conduction of electrical stimulation in the heart are far
less studied compared to electrophysiologic
mechanisms.1 Studies on the pathology of the cardiac
conduction system, summarizing key morphologic
features of the major conduction axis and the
disposition of the conduction system at a macroscopic
level, are still important contents for electrophy
siologists.2,3 There is a Korean study on the histological
findings of conduction systems.4
This review illustrates the historical sequence on the
understanding of the conduction system and its basic
anatomy, after that some questions on the anatomy of
the conduction system are formulated. Current
understanding of these issues is presented and
hypothetical answers are proposed.
Historical sequence of discovery of the conduction system of the heart
The Czech Jan Evangelista Purkynje discovered a
net of gelatinous fiber at the sub-endocardium of
ventricles in the ungulate heart and published his
discovery in Czech in 1839 and in English in 1845.5
His finding concerned the heart of an ungulate (horse
or cow), not a human heart. Little was known about its
functional significance and its histological features
were strangely interpreted as a kind of cartilage in
the heart.5 Tawara in 1906 was the first to re-discover
the significance of Purkinje cells during cardiac
contraction. Till now disagreement remains on how to
define Purkinje cells or fibers, but it is generally
understood that Purkinje cells are vacuolated cells
with fast conducting fibers at the atria or ventricular
bundle branches.
In 1893, Wilhelm His Jr. from Leipzig discovered
the atrio-ventricular bundle (His bundle) and
explained the propagation of cardiac contraction
through muscle (myogenic conduction) rather than
through nerve (neurogenic conduction), which at that
time was the dogma.5 In the same year, A.F. Stanley
Kent from Britain also described myogenic
conduction independently. Kent described the
muscular connection between atria and ventricles as
well, but he described several atrioventricular
conduction bundles (Kent bundle) in addition to the His bundle. The nodes and bundles observed by Kent
are now understood to be remnants of atrioventricular
ring tissue rather than an aberrant connection in the
Wolff-Parkinson-White syndrome.
In 1906, Sunao Tawara reported his historical
discovery of the AV node in an absolutely perfect
description. He was a Japanese physician, who
graduated from Tokyo University in 1901 and worked
with Ludwig Aschoff in Germany from 1903. His
achievements were appreciated by cardiologists not
only for his AV node discovery, but also for his
integration of previous knowledge about the
conduction system by His and Purkinje. Tawara
explained how the His bundle expands and how it
branches into the left and right bundles as described
by Purkinje. One year after Tawara s discovery, Sir
Arthur Keith and his young medical student Martin
Flack discovered the sinus node in the heart of several
species at an area that histologically resembled the
Tawara s node. In 2007, the centennial discovery of
the sinus node by Keith and Flack was celebrated.1,6
Some questions
The historical question "Why does the heart beat?"
was answered by Tawara, Keith and Flack.4,6 The next
question was on the position of the conduction
pathway in a congenitally malformed heart,
particularly in the heart with an abnormal looping
such as a congenitally corrected transposition.7,8 The
expression of ganglion nodosum, which is like an
antigen in the conduction system, was discovered by
the Amsterdam group during immuno-histochemical
studies on the molecular expression of liver.9,10 They
realized that tracing the conduction system at the
embryonic heart could reveal developmental
processes of the human heart. Studies on cell to cell
communication like gap junction proteins and on
functional development of myofilaments followed.11,12
The development of electro-physiologic techniques
to evaluate the mechanism of arrhythmia left the
question on the cellular mechanism of the abnormal
excitation of the heart10 unanswered. This question is recently studied in the pathology of the cardiac
conduction system in relation to atrial arrhythmia and
some other pathologic heart conditions such as
myocardial infarct with arrhythmia.13

Figure 1. The septal surface of the right ventricle after removal of the free anterior wall. The his bundle starts from the
atrioventricular node (AVN) and runs inferior to the border of the membranous septum (MS) and continues to the right
bundle branch (arrows) underneath the medial papillary muscle (MPM) and the septomaginal trabeculation.
CS; orifice of coronary sinus, RCA; right coronary artery, RVO; right ventricular outflow, SEP; septal leaflet of tricuspid valve, VIF; ventriculo-infundibular fold.

Figure 2. Left ventricular septal surface and anterior mitral leaflet (AML) after removal of the free wall of left ventricle
and mural leaflet of mitral valve. A wedge of fibroadipose tissue interposes at the atrioventricular junction.
ALPM; antero-lateral papillary muscle,
CS; coronary sinus, LAD; left anterior descending coronary artery, PMPM; postero-medial papillary muscle
One major group of questions left unanswered is
about the nature and functional significance of the
Purkinje cell.14 Purkinje cells are vacuolated cells at
the ventricular sub-endocardium found in the ungulate
heart. It is agreed upon that they are not seen in the
human heart. Some physicians use the term Purkinje
fibers, but others talk about Purkinje cardiomyocytes
or the Purkinje network. Sometimes, Purkinje cells are
referred to as the left bundle branch, but in other
occasions a vague term is used describing the
peripheral conduction system irrespective of its
morphology.3
Basic Anatomy
The landmark of the right ventricular aspect of the
conduction axis is documented for surgical anatomy
of the heart with ventricular septal defects, which
demonstrates its location at the inferior border of the
membranous septum of a normal heart (Figure 1), and
at the inferior border of hearts with ventricular septal
defects of a peri-membranous type. The location of
the right bundle branch is also localized at the
myocardium underneath the trabecula septomarginalis
of the right ventricle.
The left ventricular aspect of the conduction system
has a close relation with the anterior mitral leaflet
(Figure 2). The left bundle arises from the inferior
border of the membranous septum, which lies beneath
the non-coronary cusp of the aortic valve and the
anterior mitral leaflet. The medial 3/4 of the anterior
mitral leaflet is in fibrous continuity with the left cusp
and the non-coronary cusp of the aortic valve, but the
lateral 1/4 has a free wall where the left atrial and
ventricular walls are connected, having a potential for
an aberrant connection (Figure 3).
Muscle dissection
Conduction starts from the base of the ventricles
and spreads down to the apex, but the contraction
starts from the apex so that the ventricles squeeze
from the apex. 
Figure 3. Close up of the specimen Fig 2. after lifting up the anterior mitral leaflet to show the aortic valve and the
ventricular surface of the mitral anterior leaflet seen from the apex of the left ventricular cavity. Mitral-aortic fibrous
continuity is observed between the left and non-coronary cusps and the anterior part of mitral anterior leaflet (dotted
line). The posterior part of the mitral leaflet is attached both to the left atrium and left ventricle (line). Arrows indicate
the anterior and posterior sub-branches of the left bundle branch.
LC; left coronary cusp, MS; membranous septum, NC; non-coronary cusp, RC; right coronary cusp
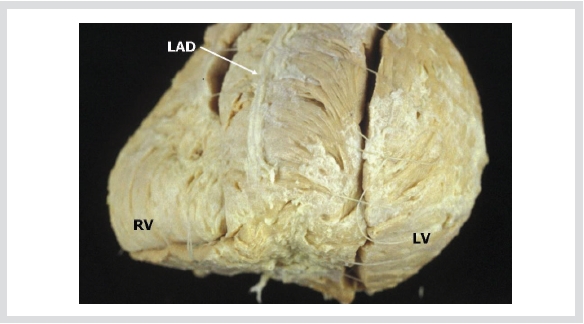
Figure 4. After removal of the epicardium and fatty tissue, we can see the direction of cardiac muscle fiber bundles,
which make whorls in the apex.
LAD; left anterior descending coronary artery, LV; left ventricle, RV; right ventricle.
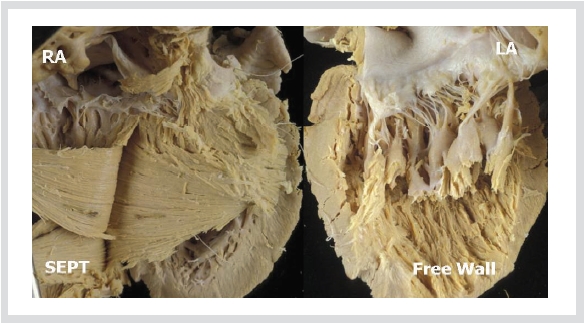
Figure 5. Further dissection of the ventricular septum shows gradual change of their direction. The free wall of the left
ventricle has a fiber direction in parallel to the long axis of heart.
Dissection of the cardiac muscle at
macroscopic level confirms this principle (Figure 4).
Then the question arises if the left and right bundle
branches form a U-turn at the apex through a counter
current of the septal area s bundle branch and the
Purkinje network (Figure 5).
Conclusion
The cardiac conduction system of ventricles
consists of the Atrioventricular node, the His bundle
and right and left bundle branches. They run to the
apex to connect to the peripheral conduction system
called Purkinje network. The histological details
and functional molecular nature of the conduction
system and Purkinje network will be discussed in part
II.
References
- Boyett MR, Dobrzynski H. The sinoatrial node is still setting the pace 100 years after its discovery. Circ Res. 2007;100:1543-1545.
- Davies MJ. Pathology of conducting tissue of the heart. London: Butterworths, 1971.
- Davies MJ, Anderson RH, Becker AE. The conduction system of the heart. London: Butterworths, 1983.
- sSeo JW, Chi JG, Lee SK. Cardiac conduction system-Morphological observations on the autopsy cases. Korean J Pathol. 1983;17:127-137.
- James TN. The development of ideas concerning the conduction system of the heart. Ulster Med J. 1982;51:81-97.
- Silverman ME, Hollman A. Discovery of the sinus node by Keith and Flack: on the centennial of their 1907 publication. Heart. 2007;93:1184-1187.
- Lev M, Fielding RT, Zaeske D. Mixed Levocardia with Ventricular Inversion (Corrected Transposition) with Complete Atrioventricular Block. A Histopathologic Study of the Conduction System. Am J Cardiol. 1963;12:875-883.
- Anderson RH. The conduction tissues in congenitally corrected transposition. Ann Thorac Surg. 2004;77:1881-1882.
- Wessels A, Vermeulen JL, Verbeek FJ, Viragh S, Kalman F, Lamers WH, Moorman AF. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992;232:97-111.
- Moorman AF, Christoffels VM, Anderson RH. Anatomic substrates for cardiac conduction. Heart Rhythm. 2005;2:875-886.
- Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol. 1995;6:813-822.
- van Kempen MJ, Fromaget C, Gros D, Moorman AF, Lamers WH. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ Res. 1991;68:1638-1651.
- de Bakker JM, van Rijen HV. Electrocardiographic manifestation of anatomical substrates underlying post-myocardial infarction tachycardias. J Electrocardiol. 2007;40:S21-25.
- Di Maio A, Ter Keurs HE, Franzini-Armstrong C. T-tubule profiles in Purkinje fibres of mammalian myocardium. J Muscle Res Cell Motil. 2007;28:115-121.
|