|
Introduction
Since the first direct left atrial pressure recording
in a patient with an atrial septal defect by Cournand
et al.,1 different approaches such as the transbronchial2
and percutaneous apical left ventricle
puncture3 methods have been used for obtaining the
pressure of the left cardiac chamber in order to treat
patients with various heart diseases. The current
transseptal catheterization was first performed by
Ross et al .,4 and the technique underwent several
modifications since then, to meet the specific needs.5-7
Transseptal catheterization has been used for
diagnostic purposes in valvular heart disease,
however with the development of new non-invasive
technologies such as 2-dimentional (D) echo/Doppler
and cardiac MRI, the need for transseptal catheterization
has greatly decreased since early 1990. The interest
in the transseptal catheterization was revived by
interventional cardiology in order to meet the needs
of the new percutaneous therapeutic procedures
such as mitral and aortic valvuloplasty, closure of
atrial septal defects, left atrial appendage occlusion,
and the recent mitral annuloplasty.
Transseptal catheterization was also revived by
the interventional electrophysiology field and has
been used to treat a variety of arrhythmias including
left sided accessory pathways,8 left atrial or
pulmonary vein origin atrial tachycardias,9, 10 atrial
fibrillation,11 and ventricular tachycardia.12 Thus, the
transseptal catheterization technique was further
modified in a unique way, and currently several
transseptal techniques have been reported in the
literature.13-17 With the recent advances in atrial
fibrillation ablation, the double transseptal
catheterization technique has become the
cornerstone of atrial fibrillation ablation. Therefore,
a proper transseptal catheterization is essential for a
successful left atrial or ventricular arrhythmia
ablation.
This article intended to review some of the
important considerations for transseptal catheterization
in the cardiac electrophysiology practice for patients
with left atrial and ventricular arrhythmias.
Anatomical considerations
A comprehensive review of the normal atrial
anatomy has been reported in the literature,18-20 and it
is important to review that literature prior to
mastering the technique. The atrial septum, in
particular the anatomy of the fossa ovalis is the
primary interest for the transseptal procedure. The
fossa ovalis has an average diameter of 21 mm and has a well defined distinctive limbic ledge in its
superior, anterior and inferior margins. The limbic
ledge has been used as the landmark for the
transseptal left heart catheterization.7 The posterior
margin is not well defined due to the lack of a
distinctive ledge and has a gradual transition of the
tissue thickness from a thick inferoposterior septum
to a thin anterosuperior membrane (Figure 1). The
clinical implications of these anatomical characteristics
is that it would be much easier to engage the
transseptal apparatus (sheath and needle) on the
fossa ovalis with a posterior to anterior sweeping
motion than with an anterior to posterior motion.
This maneuver also avoids the confusion between
the fossa ovalis and the crevices that often present in
the anterior atrial septum which are located just
opposite to the aorta.
Another important structure that should be
recognized is the superior vena cava (SVC)- aorta
groove. The SVC-aorta groove is formed by the
most posterior margin of the ascending aorta and
posteromedial wall of the SVC. During the
craniocaudal dragging of the transseptal apparatus,
the groove is easily recognized by rotating the
transseptal apparatus in the horizontal orientation
within the mid-SVC. The groove is located at the
most medial portion of the SVC in left anterior
oblique (LAO) projection of the fluoroscopy and the
lower part of the groove leads into the fossa ovalis.
Clinical considerations
There are many variations in the cardiac anatomy
among patients and those variations can directly
influence the transseptal catheterization. As the
patient population grows older, deformities of the vertebrae and chest wall become much more
frequent among them.

Figure 1. Anatomy of the fossa ovalis. Panel A and B show a right lateral view of the atrial septum to illustrate the
fossa ovalis. The fossa ovalis is bordered by the limbic ridge in its superior and anterior aspects and by the eustasian
ridge in its the infero-anterior aspect. There is no clear border in infero-posterior aspect of it (black arrows) that may
have a significant implication during the transseptal catheterization.
The deformities of the chest
wall including the sternum or vertebrae can often
lead to anatomical changes in not just the heart, but
also the major vessels. For example, severe scoliosis
of the thoraco-lumbar vertebrae can create a
significant difficulty in applying proper torque
during the transseptal catheterization and it is
impossible to perform the transseptal catheterization
from the femoral veins (Figure 2A). However, if the
patient has severe rightward scoliosis, the
transseptal catheterization can be successfully
performed from the left femoral vein. Thus, a
comprehensive clinical examination is very
important to appreciate these anatomical variations,
especially those related to the chest or torso.
Diseases of the great arteries such as an ascending
aortic aneurysm or underlying lung disease can also
alter the cardiac anatomy including the atrial septum
orientation that directly influences the transseptal
catheterization. It is also important to evaluate the
patients with particular attention paid to the
presence of a large hiatal hernia that could deform
the posterior cardiac border and increase the risk for
life threatening complications.
Three-D imaging such as computed tomography
(CT) angiography or magnetic resonance imaging
(MRI) are extremely helpful for evaluating the atrial septal/fossa ovalis anatomy, patency of the proximal
vessels, and vital adjacent non-cardiac structures
especially in patients with an inferior vena cava
(IVC) filter (Figure 2B, 2C) for a previous
pulmonary embolism or the presence of a large
hiatal hernia. Furthermore, 3-D imaging can
diagnose unsuspected congenital defects such as
atrial septal defects including the venous type, a
persistent left SVC, or the total absence of an IVC.
Therefore, it allows clinicians to plan and select the
proper transseptal approach (femoral versus neck
approach,21, 22 Figure 3) or another alternative therapy.
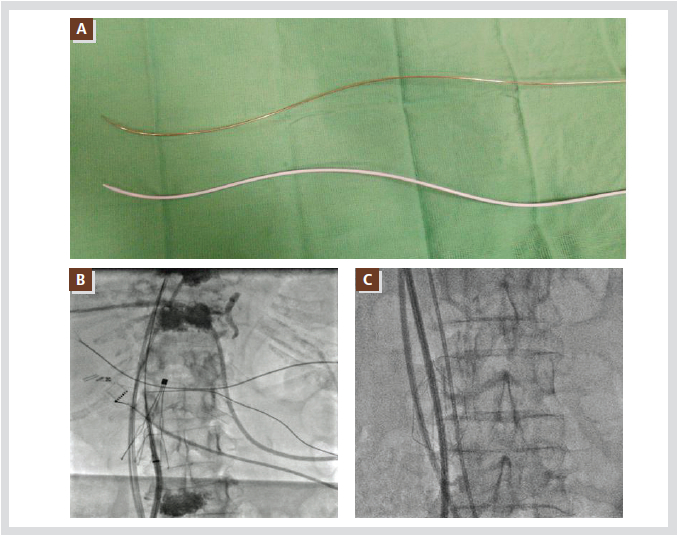
Figure 2. Examples of anatomical barriers for the transseptal puncture. Panel A shows a modified shape of the
transseptal needle and dilator in a patient with severe scoliosis. Panels B and C show the different shaped IVC filters,
which require caution when passing the transseptal sheaths. All abbreviations are as used in the text.
Technological considerations
1. Transseptal sheaths and dilators
Several companies manufacture different shapes
of transseptal sheaths (Figure 4A). They can vary in
length as well as in stiffness. There are braided and
non-braided sheaths. The braided kind is much
stiffer than the non-braided and can provide much
more support during the puncture of the fossa ovalis
(Figure 4A 3,4). Therefore, the braided sheaths are
useful for puncturing a thicker fossa ovalis and for applying greater torque to the ablation catheter.
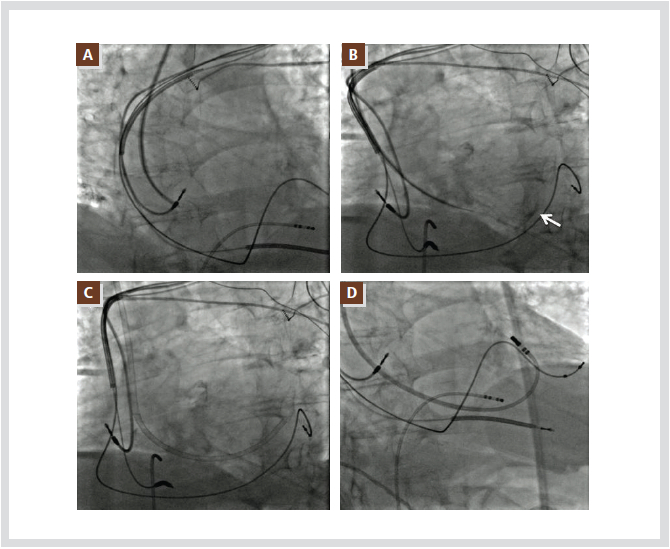
Figure 3. Shows a transseptal puncture through the internal jugular vein due to a thrombotic obstruction of the IVC.
The transseptal sheath is advanced into the interatrial septum and a sudden drop at the limbic ledge can be seen (A).
After passing the needle, a contrast agent reveals the lower left atrial wall (B, white arrow). The transseptal sheath can
be placed in the LA over the needle and dilator (C), and mapping and ablation can be performed successfully (D). All
abbreviations are as used in the text.
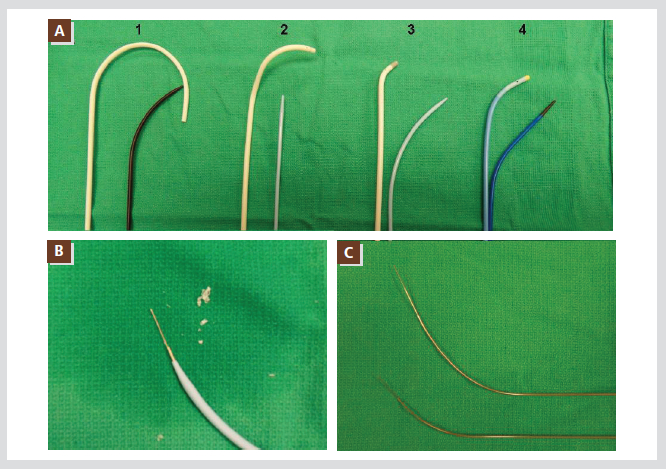
Figure 4. Panel A shows the most commonly used transseptal sheaths and dilators; Mullin sheath (1), SL-2 sheath (2),
SL-1 sheath (3), and Preface sheath (4). The braided sheath has different colors at its tip (3,4). Panel B shows the
material scraped from the dilator by the transseptal needle. There are several manufactured transseptal needles with
different shapes and lengths (C). All abbreviations are as used in the text.
The
most popular, commonly used sheaths were
developed by Dr. John Swartz in the early 1990’s to
target the variety of left sided accessory pathways
and those sheaths are quite useful for the ablation of
left atrial and ventricular arrhythmias including
atrial fibrillation. Some of them however need to be
exchanged after performing each successful
transseptal catheterization with a traditional
Mullin’s sheath due to its unique shape. The
majority of the centers use these sheaths as part of
the primary apparatus for performing the transseptal
procedure and this eliminates the need for an
exchange, and results in cost savings, a reduced
procedure time, and a lower complication rate. The
most widely used sheaths are the SL-1 (Fast-Cath
guiding introducer, St. Jude Medical, Inc., St. Paul,
MN, USA) and Preface® (Biosense Webster, Inc.,
Irwindale, CA, USA), and it is possible to reach
more than 99% of the target sites on the left side
with those sheaths. Recently several companies
have developed deflectable guiding sheaths (9.5 F to
11 F) that can be exchanged after the initial
transseptal catheterization with a smaller sheath.
These sheaths have physical characteristics of being
large in diameter and significantly stiffer than the
conventional standard 8 to 8.5 F sheaths. We do not
use these sheaths routinely in our laboratory due to the increased risk of perforation, groin complications,
and costs. They are reserved for patients with an
extremely difficult anatomy that standard sheaths
fail to allow reaching the target area.
The dilators are provided with the sheath and they
also have a wide range of physical characteristics
that may become important during the transseptal
catheterization. In particular, the dilator’s stiffness
and inner lumen diameter are the most important
factors for a safe needle placement. We observed a
significant incidence in scrapping the inner surface
of the dilator during the transseptal needle insertion.
This scrapping is due to a mismatch the size of the
needle and dilator (Figure 4B). The scrapping will
result in a difficult protruding of the needle beyond
the dilator tip during the puncture, and an early
thrombus formation may occur. To avoid this
problem, we recommend pre-loading the transseptal
needle into the dilator to test the proper match
between the dilator and needle before inserting it
into the patient’s body.
2. Transseptal needles
There are several manufactured transseptal
needles currently available. They can vary in shape
and length (Figure 4C). The proper needle curvature
is also essential to avoid any complications and to
achieve an ideal transseptal puncture.
3. X-ray equipment
Many cardiac catheterization laboratories are
equipped with a full range of motion (180。)
fluoroscopy. The interventional cardiologist often
uses bi-plane cine fluoroscopy to perform transseptal
punctures using anteroposterior (AP) and left lateral
views. In our laboratory, we use single plane cine
fluoroscopy and the usual range of fluoroscopy is
between RAO 45 and LAO 45. The fluoroscopic angles
for each patient vary depending on the orientation of
the atrial septum.
4. Transesophageal echocardiography (TEE) or
intracardiac echocardiography (ICE)
TEE: Because of the reguired intubation to protect
the airway during the early experience with this
technique and the resulting discomfort for the
patient, routine use of TEE become quickly
unpopular. The TEE probe blocks the view of the
upper region of the left atrium (LA), and the inferior
pulmonary veins cannot be visualized well.
ICE: Many institutions in the US and Europe have
been practicing the routine use of ICE to guide the
transseptal catheterization. Obviously, the use of
ICE adds an additional safety margin by confirming
the engagement of the transseptal apparatus into the
fossa ovalis, and can offer early detection of
complications such as a hemopericardium.
However, due to the small real time 2-D visual
field, it often cannot confirm the ideal puncture site
within the fossa ovalis. Furthermore, routine use in
developing countries adds a high additional cost and
requires a minimum of a 9F sheath to place the ICE
catheter which can cause more vascular and
bleeding complications. We have reserved the use of
ICE for complex congenital cardiac patients in order
to guide not just the transseptal procedure, but also
to evaluate the complex cardiac anatomies.
Transseptal catheterization
1. Anticoagulation of pre-transseptal catheterization
Generally, no anticoagulation is used prior to the
transseptal catheterization except for chronic atrial
fibrillation patients with a high risk of thromboembolisms. There are two practical options to
consider for these high risk patients. The first is the
continuous administration of oral anticoagulants
until the procedure day. The second option is to
discontinue warfarin 3 days before the procedure
and bridge that time with low molecular weight
heparin. Currently, many centers in the US do not
stop the oral anticoagulants before the atrial
fibrillation ablation and the procedure is performed
with an INR level between 2.0~2.5. This can avoid
any inconvenience, such as bridging with low
molecular weight heparin before and after the
procedure, and can be cost saving for the patients.
We have been using a continuous oral
anticoagulation strategy for the last year and it has
improved the patient’s comfort without increasing
the incidence of bleeding complications such as a
hemopericardium. This approach is particularly
useful in patients with mechanical heart valves, and
can shorten their hospital stay.
2. Direct pressure monitoring
We do not use routine arterial cannulation for
blood pressure monitoring during the transseptal
catheterization, but the majority of the ablation
centers prefer direct arterial pressure monitoring.
However, during the transseptal puncture, we
monitor the pressure from the transseptal apparatus
directly to confirm the left atrial access. Therefore,
only one pressure monitor system is used.
3. Heparinization during the transseptal
catheterization
We do not heparinize the patients until the
transseptal catheterization is completed but an
exception is considered in cases when the second
transseptal puncture is delayed for more than 15
minutes. We have not observed a significant
increase in the thromboembolic complications
related to transseptal catheterizations but some
centers have been using a heparinization strategy
prior to the transseptal catheterization. The usual
recommended heparin loading is 100 unit/Kg of
bodyweight.
4. Transseptal catheterization techniques
Before the transseptal catheterization, we
recommend always to obtain a baseline cine image
of the motion of the left cardiac border in the LAO
projection so that one can compare it throughout the
procedure. This will allow identifying any
significant pericardial fluid accumulation and may
prevent a full-blown, hemodynamically unstable
pericardial tamponade.
There are several different techniques for
performing a transseptal catheterization in terms of
the number and shape of the sheaths used, and the
number of actual transseptal punctures which varies
from one to three.14, 23 We employ a technique with
separate double punctures using braided SL-1 (Fast-
Cath guiding introducer, St. Jude Medical, Inc., St.
Paul, MN, USA) and Preface® (Biosense Webster,
Inc., Irwindale, CA, USA) sheaths and a standard
size transseptal needle that can be shaped according
to the patient’s atrial anatomy (size or volume). A
longer sheath and needle are required for patients
with a large body mass index (weight >125 Kg,
height >190.5 cm), but such patients are rare among
Asian populations except for acromegalic patients
who are known to develop atrial fibrillation. The
transseptal sheaths need to be prepared carefully
before insertion into the patient’s vascular space,
and special attention is needed to avoid scrapping
the dilator while introducing the transseptal needle.
We believe that these scrapped-off plastic materials are highly thrombogenic and possibly
responsible for the early thrombus formation
observed in ICE studies during transseptal
catheterizations.
The sequence of the transseptal catheterization is
shown in Figure 5. The stepwise techniques are as
follows:
1) Place the guide wire into the left subclavian
vein.
2) Advance the sheath into the left subclavian vein
over the guide wire to avoid inadvertent SVC
injury or perforation.
3) Remove the dilator and guide wire.
4) Aspirate and flush the sheath to eliminate any
dead space within the sheath.
5) Load the transseptal needle with the stylet into
the dilator on the preparation table to test for a
smooth transition without any scraping. Then,
remove the stylet and the stopcock of the
needle hub should be locked after flushing.
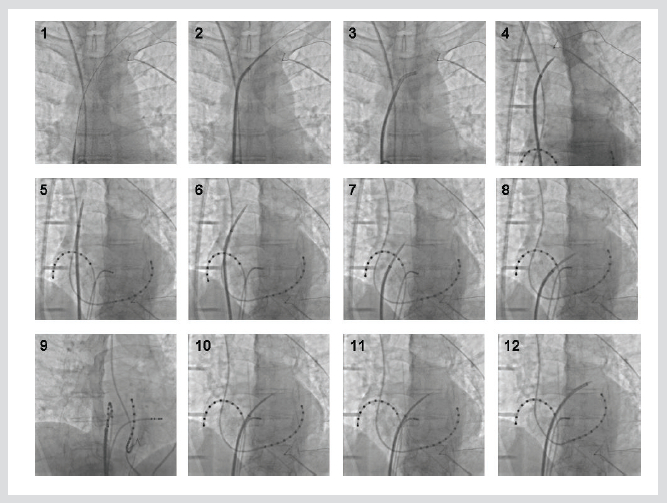
Figure 5. Shows the transseptal catheterization sequence. 1) Place the guide wire inside of the left subclavian vein. 2)
Advance the sheath into the left subclavian vein over the guide wire. 3) Remove the dilator and guide wire. 4) Advance
the dilator with the needle inside. When the dilator approaches the tip of the sheath, pull the sheath back and lock it to
the dilator. 5~6) Rotate the transseptal apparatus anterior and posteriorly to find the most medial direction. 7~8)
Being dragged continuously, the transseptal apparatus will move medially (toward the LA) with a sudden distinctive
leftward motion. 9) The ideal puncture site for AF ablation is a distance of 40% from the posterior margin of the left
atrium and 60% from the CS catheter. 10) The transseptal needle is advanced toward the left main bronchus in the
LAO view while monitoring the pressure. 11~12) Once a successful puncture of the fossa ovalis is confirmed, then the
dilator with the sheath is advanced over the needle, and the dilator with needle is removed. All abbreviations are as in
used the text.
6) Load the dilator with the needle inside into the
sheath which has already been placed in the left
subclavian vein. When the dilator approaches
the tip of the sheath, pull the sheath back and
lock it to the dilator to avoid inadvertent vessel
injury from advancing the dilator.
7) The transseptal apparatus (sheath, dilator and
needle) is flushed before connecting it to the
pressure monitor system.
8) The transseptal apparatus is withdrawn
gradually and pointed toward the atrial septum
in the LAO view (recommended LAO angle:
the His bundle catheter is pointing directly
toward the operator). It is important to identify
the SVC-aortic groove that is formed by the
posterior margin of the ascending aorta. The
best region to identify the groove is the midlevel
of the SVC, and the groove is located at
the most medial portion of the SVC in the LAO
view which can be identified by rotating the
transseptal apparatus anteriorly or posteriorly
(clockwise or counter-clockwise motions).
9) Being dragged continuously, the transseptal
apparatus will move medially (toward the LA)
with a sudden distinctive leftward motion when
it engages into the fossa ovalis and this
distinctive leftward motion of the transseptal
apparatus is appreciated better in the LAO view
than the PA or right anterior oblique (RAO)
views. Occasionally, some patients have large
sized crevices at the anterior atrial septum
which can be confused for the fossa ovalis.
This can be differentiated from the fossa ovalis
confirming in the RAO view that the
transseptal apparatus is in the posterior septum:
fossa ovalis.
10) The final adjustment is done in the RAO view
(recommended RAO angle: coronary sinus
(CS) catheter is perpendicular to the operator)
where one can appreciate the anterior margin
(CS catheter) and posterior margin of the
cardiac silhouette.
11) The transseptal apparatus can be rotated
anteriorly or posteriorly to reach the final
puncture site that the operator desires within
the fossa ovalis. The ideal puncture site is
illustrated in Figure 6 (a distance of 40% from
the posterior margin of the LA and 60% from
the CS catheter).
12) The transseptal needle is advanced toward the
left main bronchus in the LAO view while
monitoring the pressure to confirm the
puncture of the fossa ovalis. If there’s no
immediate LA pressure recording after the
puncture, a small volume of contrast agent is
injected through the needle to confirm the
needle position in relation to the septum or
LA cavity.
13) An inadvertent puncture with the transseptal
needle (but not the dilator) of undesirable sites
such as the ascending aorta, septum or
posterior wall have not been associated with
major complications.
14) Once a successful puncture of the fossa ovalis
is confirmed then the dilator with the sheath is
advanced over the needle and the dilator with
the needle is removed.
15) Approximately 5~6 mL of blood is aspirated
from the sheath and is then flushed.
16) The 2nd transseptal puncture can be placed at a
site 5 ~10 mm anteroinferior or posterosuperior
from the first puncture site (Figure 7). A
posterior puncture is essential for accessing
the low posteriorly located right inferior
pulmonary vein and inferoposterior region of
LA. The anterior puncture is for the anteriorly
located superior pulmonary veins of LA
during the procedure.
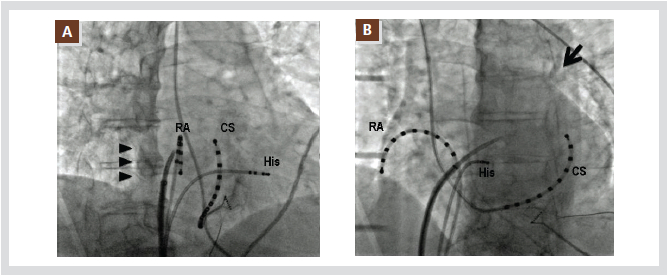
Figure 6. Shows the ideal transseptal puncture site for atrial fibrillation ablation. The recommended RAO angle is that
with the CS catheter perpendicular to the operator (A). In the RAO projection, the distance between the posterior margin
of the LA (arrow head) and CS catheter can be easily measured. The ideal puncture site for AF ablation is a distance of
40% from the posterior margin and 60% from the CS catheter in the RAO view. The recommended LAO angle is that
with the His bundle catheter pointing directly toward the operator (B). After engagement of the transseptal apparatus
into the fossa ovalis, the apparatus should be advanced toward the left main bronchus (arrow).
RA: right atrium. The other abbreviations are as use in the text.
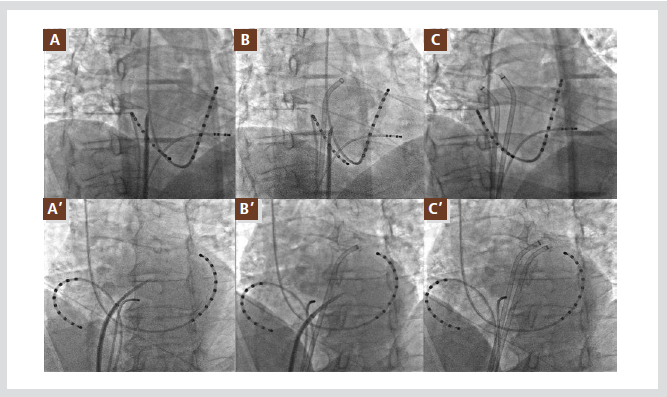
Figure 7. Shows an anteroinferior and superoposterior double transseptal puncture. The first transseptal puncture site
is selected at a superior and posterior site of the fossa ovalis (A, A’). The 2nd transseptal puncture can be placed at a site
5 ~ 10 mm anteroinferior from the first puncture site (B, B’). The two well separated transseptal sheaths can provide
various accesses to the pulmonary veins (C, C’). The top row shows the RAO projection of the fluoroscopy and bottom
row the LAO projection of each step. All abbreviations are as used in the text.
A few techniques for specific conditions
1. Patent foramen ovale (PFO)
We do not use a PFO as an access for the
transseptal approach for atrial fibrillation ablation
due to the lack of septal support for the transseptal
sheath, which causes the catheter placement to be
unstable. Thus, the transseptal sheath is placed
through a puncture of the intact portion of the fossa
ovalis.
2. The accidental migration of the transseptal sheath
into the right atrium
The transseptal sheath and ablation catheter can
be accidently or purposely pulled back into the right
atrium during the ablation procedure, especially for
ablation of the right pulmonary veins. In the
majority of cases, the sheath can be placed back
through the same puncture site using the ablation
catheter.
3. Difficulty in manipulating the transseptal sheath
and ablation catheter
From time to time, we encounter a significant
difficulty in manipulating the ablation catheter and
transseptal sheath during the procedure. It is often
due to an undesirable transseptal puncture site: too
anterior from the ideal site. We recommend a
careful evaluation of the transseptal puncture site,
and a re-puncture at an ideal site as soon as the
problem is identified. This will minimize the
procedure time and avoid complications.
4. Thick membrane of the fossa ovalis
Occasionally, we encounter patients who have a
myxomatous membrane of the fossa ovalis that can
be quite thick and cannot be punctured with the
standard needle. Recent studies report that it is
helpful and safe to use a radiofrequency current
delivery from a standard electrocautery device in
conjunction with a standard transseptal needle in
order to make a small hole in the atrial septum and
then advance the transseptal apparatus.24, 25 This
approach can be useful in failed standard transseptal
punctures, but we do not use it in routine practice.
Special conditions
It is not uncommon to encounter patients who
have received several cardiovascular devices for the
treatment of underlying diseases and have been
referred for a catheter ablation that requires
transseptal catheterization.
1. Pacemakers and implantable cardioverter
defibrillators (ICDs)
The most common device that we encounter
during the transseptal catheterization is a pacemaker
or ICD. The transvenous leads of these devices can
often create obstacles during the transseptal
catheterization. The transseptal apparatus needs to
be medial to the leads in the LAO view in order to
avoid entanglement (Figure 8A).
2. Septal closure devices
There are two major brands of septal closure
devices that have been implanted in the atrial
septum for atrial septal defects (ASDs) or PFOs.26
Each one has different sizes and shapes. A careful
review of the implant record prior to the procedure
is essential and an ICE-guided transseptal puncture
is recommended. The common successful puncture
site is the inferoposterior margin of the closure
device (Figure 8B), and no more than one
transseptal sheath should be placed.
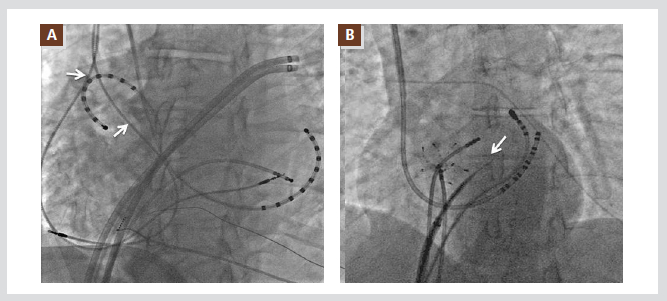
Figure 8. Special conditions to consider during the transseptal puncture. The transseptal apparatus needs to be medial
to the pacemaker or ICD leads (arrows) in the LAO view in order to avoid entanglement with the leads (A). The patients
with atrial septal occluders can be candidates for a septal puncture. A common successful puncture site is the
inferoposterior margin of the closure device (arrow in B). All abbreviations are as used in the text.
3. ASD surgical repairs
Patients, who have undergone a surgical ASD
repair and have an absolute need for a transseptal
catheterization, should be referred to an experienced
center for this type of patient. In our experience,
Dacron grafts are the most difficult structures to
penetrate with the transseptal needle and the sheath,
and catheter manipulation is extremely limited and
difficult. Pericardial patches can be accessed with a
standard needle but the success rate of the puncture
appears to get lower as the patch gets older due to
the extensive scarring and fibrosis of the patch.
4. IVC filters
It is not uncommon to encounter patients with an
IVC filter in our practice. Most IVC filters can be
crossed safely with electrophysiological catheters
and transseptal sheaths. All long sheaths should be
passed over the guide wire with the dilator with
caution, under fluoroscopic visualization during the
insertion as well as during the removal (Figure 2B,
C).
These patients may also have significant femoral
or iliac vein sclerosis from previous thromboembolic
events that can result in an unsuccessful femoral
vein cannulation. The IVC filter may limit the
transseptal apparatus manipulation during the
transseptal puncture but there have been no major
difficulties in the majority of cases.
Conclusions
The transseptal catheterization is becoming an
integral part of the interventional electrophysiology
practice. However, the transseptal catheterization
technique used in electrophysiology is being
differentiated from interventional cardiology due to
the different purposes and objectives of the
procedures. Therefore, it is important to understand
the atrial anatomy, especially the interatrial septum,
completely before practicing the transseptal
catheterization. The transseptal catheterization can
be practiced safely and efficiently for the treatment
of cardiac arrhythmias.
References
- Cournand A, Motley HL, et al. Recording of blood pressure from the left auricle and the pulmonary veins in human subjects with interauricular septal defect. Am J Physiol. 1947;150:267-271.
- Facquet J, Lemoine JM, Alhomme P, Lefebvre J. [transbronchial measurement of left auricular pressure.]. Arch Mal Coeur Vaiss. 1952;45:741-745.
- Brock R, Milstein BB, Ross DN. Percutaneous left ventricular puncture in the assessment of aortic stenosis. Thorax . 1956;11:163-171.
- Ross J, Jr. Catheterization of the left heart through the interatrial septum: A new technique and its experimental evaluation. Surg Forum. 1958;9:297-300.
- Brockenbrough E, C, Braunwald E, Ross J, Jr. Transseptal left heart catheterization: A review of 450 studies and description of an improved technic. Circulation. 1962;25:15-21.
- Mullins CE. Transseptal left heart catheterization: Experience with a new technique in 520 pediatric and adult patients. Pediatr Cardiol. 1983;4:239-245.
- Bloomfield DA, Sinclair-Smith BC. The limbic ledge. A landmark for transseptal left heart catheterization. Circulation . 1965;31:103-107.
- Swartz JF, Tracy CM, Fletcher RD. Radiofrequency endocardial catheter ablation of accessory atrioventricular pathway atrial insertion sites. Circulation. 1993;87:487-499.
- Kay GN, Holman WL, Nanda NC. Combined use of transesophageal echocardiography and endocardial mapping to localize the site of origin of ectopic atrial tachycardia. Am J Cardiol. 1990;65:1284-1286.
- Tracy CM, Swartz JF, Fletcher RD, Hoops HG, Solomon AJ, Karasik PE, Mukherjee D. Radiofrequency catheter ablation of ectopic atrial tachycardia using paced activation sequence mapping. J Am Coll Cardiol. 1993;21:910-917.
- Swartz JF, Perrersels G, Silvers J. A catheter based curative approach to atrial fibrillation in humans. Circulation. 1994;90.
- De Ponti R, Zardini M, Storti C, Longobardi M, Salerno-Uriarte JA. Trans-septal catheterization for radiofrequency catheter ablation of cardiac arrhythmias. Results and safety of a simplified method. European Heart Journal. 1998;19:943-950.
- Fagundes RL, Mantica M, De Luca L, Forleo G, Pappalardo A, Avella A, Fraticelli A, Dello Russo A, Casella M, Pelargonio G, Tondo C. Safety of single transseptal puncture for ablation of atrial fibrillation: Retrospective study from a large cohort of patients. J Cardiovasc Electrophysiol. 2007;18:1277-1281.
- Yamada T, McElderry HT, Epstein AE, Plumb VJ, Kay GN. Onepuncture, double-transseptal catheterization manoeuvre in the catheter ablation of atrial fibrillation. Europace. 2007;9:487- 489.
- Haruta S, Kouno H, Akanuma H, Ichinose H, Shimakura T. The guidewire technique for transseptal puncture. J Invasive Cardiol. 2005;17:68-70.
- Daoud EG, Kalbfleisch SJ, Hummel JD. Intracardiac echocardiography to guide transseptal left heart catheterization for radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1999;10:358-363.
- Roberto De P, Riccardo C, Antonio C, Paolo Della B, Luigi P, Antonio R, Massimo S, Jorge AS-U. Trans-septal catheterization in the electrophysiology laboratory: Data from a multicenter survey spanning 12 years. J Am Call Cardiol. 2006;47:1037- 1042.
- Anderson RH, Webb S, Brown NA. Clinical anatomy of the atrial septum with reference to its developmental components. Clin Anat. 1999;12:362-374.
- Anderson RH, Brown NA, Webb S. Development and structure of the atrial septum. Heart. 2002;88:104-110.
- Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: Implications for radiofrequency
- Bevegard S, Carlens E, Jonsson B, Karlof I. A technique for transeptal left heart catheterization via the right external jugular vein. Thorax. 1960;15:299-302.
- Epstein EJ, Coulshed N. Transseptal catheterization via right subclavian vein. Br Heart J. 1971;33:658-663.
- Takashima H, Kumagai K, Matsumoto N, Yasuda T, Nakashima H, Yamaguchi Y, Hida S, Muraoka S, Mitsutake C, Miura S-I, Saku K. Characteristics of the conduction of the left atrium in atrial fibrillation using non-contact mapping. Journal of Cardiology. 2010;56:166-175.
- Bidart C, Vaseghi M, Cesario DA, Mahajan A, Fujimura O, Boyle NG, Shivkumar K. Radiofrequency current delivery via transseptal needle to facilitate septal puncture. Heart Rhythm. 2007;4:1573-1576.
- Capulzini L, Paparella G, Sorgente A, de Asmundis C, Chierchia GB, Sarkozy A, Muller-Burri A, Yazaki Y, Roos M, Brugada P. Feasibility, safety, and outcome of a challenging transseptal puncture facilitated by radiofrequency energy delivery: A prospective single-centre study. Europace. 2010;12:662-667.
- Crystal MA, Ing FF. Pediatric interventional cardiology: 2009. Curr Opin Pediatr. 2010;22:567-572.
|