Efficacy and Transesophageal Echocardiographic Findings of Short-term Dabigatran or Rivaroxaban Therapy for Electrical Cardioversion in Patients with Atrial Fibrillation
Article information
Abstract
Background and Objectives:
The efficacy of short-term novel oral anticoagulant (NOAC) therapy for electrical cardioversion has not been fully evaluated. This study sought to investigate the efficacy and transesophageal echocardiographic (TEE) findings of short-term dabigatran or rivaroxaban therapy versus warfarin therapy in patients undergoing electrical cardioversion for atrial fibrillation.
Subject and Methods:
This registry study included patients with persistent atrial fibrillation who underwent elective electrical cardioversion. We analyzed 115 patients (42 in dabigatran, 33 in rivaroxaban, and 40 in warfarin group) who were taking oral anticoagulants for maximum of 42 days. All patients underwent TEE before electrical cardioversion. The primary outcome was stroke or systemic embolism at 30 days. Another outcome was major bleeding.
Results:
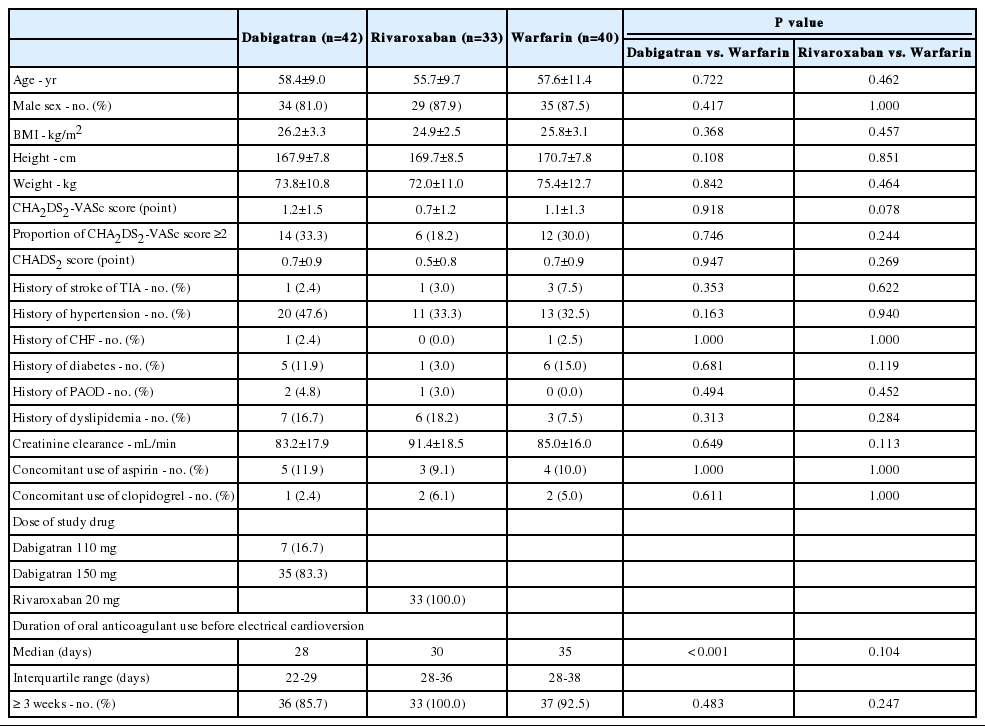
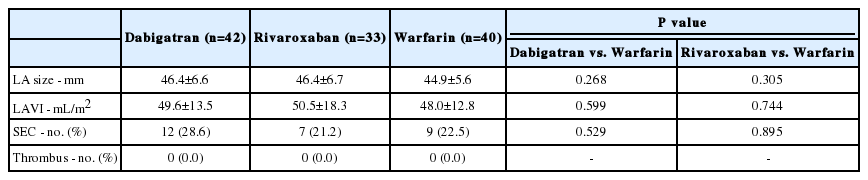
Baseline characteristics were similar between patients in the NOAC and warfarin groups. The mean CHA2DS2-VASc score was 1.2±1.5 in the dabigatran group, 0.7±1.2 in the rivaroxaban group, and 1.1±1.3 in the warfarin group (p=0.918, dabigatran vs. warfarin; p=0.078, rivaroxaban vs. warfarin). None of the patients in this study had thrombi. There was no difference in the incidence of left atrial spontaneous echo contrast between groups (28.6%, 21.2%, and 22.5% in dabigatran, rivaroxaban, and warfarin groups, respectively; p=0.529, dabigatran vs. warfarin; p=0.895, rivaroxaban vs. warfarin). No strokes or systemic embolisms were observed during the 30-day follow-up period. There were no major bleeding events.
Conclusion:
The short-term use of dabigatran or rivaroxaban can be considered safe in patients undergoing electrical cardioversion for atrial fibrillation.
Introduction
Because maintenance of sinus rhythm is often associated with improved quality of life and exercise performance, restoration and maintenance of sinus rhythm remains the major goal in patients with atrial fibrillation [1]. Electrical cardioversion can restore sinus rhythm in patients with atrial fibrillation, although this procedure is associated with an increased risk of thromboembolic events [2-4]. If therapeutic anticoagulation is inadequate, this risk is higher (5% to 7%) [5,6], but adequate anticoagulation reduces the risk of thromboembolic events (0.7% to 0.8%) [7]. Therefore, current guidelines recommend therapeutic anticoagulation with warfarin for 3 weeks before and 4 weeks after cardioversion [8,9]. Novel oral anticoagulants (NOACs) have been introduced as alternatives to vitamin K antagonists. Several post-hoc analyses have evaluated the efficacy and safety of NOACs in the setting of cardioversion [10-12]. However, these studies either did not report the length of NOAC use or included patients who had been using them for an extended period of time, preventing an evaluation of the safety of short-term NOAC therapy before cardioversion for atrial fibrillation. This study investigated the safety and transesophageal echocardiographic (TEE) findings of short-term NOAC therapy versus warfarin therapy before electrical cardioversion in patients with atrial fibrillation.
Subjects and Methods
Study Population
This registry study included patients with persistent atrial fibrillation who underwent elective electrical cardioversion at Samsung Medical Center from January 1, 2012 to May 30, 2014. Eligible patients had atrial fibrillation documented on electrocardiography performed before and on the day of electrical cardioversion. We enrolled patients who had been taking dabigatran, rivaroxaban, or warfarin for maximum of 42 days before electrical cardioversion. Key exclusion criteria included atrial fibrillation due to a reversible cause, moderate or severe mitral stenosis, and conditions other than atrial fibrillation that required anticoagulation. All patients maintained therapeutic anticoagulation for at least 30 days after electrical cardioversion.
Study Design and Endpoints
Demographic data, cardiovascular risk factors, medications, and causes of electrical cardioversion were analyzed by reviewing medical records. Patients were divided into three groups according to the oral anticoagulant used (dabigatran, rivaroxaban, or warfarin group).
The primary endpoints were stroke or systemic embolism after electrical cardioversion. Another outcome was major bleeding. All patients were monitored for the first 30 days after electrical cardioversion to evaluate thromboembolic events and bleeding complications.
We defined stroke as the sudden onset of a focal neurological deficit in a location consistent with the territory of a major cerebral artery, confirmed by imaging techniques. Systemic embolism was defined as an acute vascular occlusion of an extremity or an organ, documented by means of imaging, surgery, or autopsy. Major bleeding was defined as a reduction in the hemoglobin level of at least 20 g/L, transfusion of at least 2 U blood, or symptomatic bleeding in a critical area or organ. All other bleeding was considered minor.
Transthoracic and TEE Parameters
All patients underwent TEE within 3 months of electrical cardioversion. Left atrial (LA) diameter, LA volume index, and left ventricular ejection fraction were estimated. TEE was also performed in all patients to evaluate spontaneous echo contrast (SEC) or thrombi before electrical cardioversion.
Statistics
Continuous data are expressed as mean ± standard deviation or median and interquartile range. Categorical data are expressed as frequency and percentage. To evaluate differences between study groups, we used the Student’s unpaired t test for normally distributed data and the Mann-Whitney U test for skewed data. Categorical data were analyzed with chi-squared or Fisher’s exact tests. We used SPSS software (SPSS for Windows, version 20.0, IBM Corp., Armonk, NY, USA). A P value< 0.05 was considered to be significant.
Results
Baseline Clinical Characteristics of the Study Population
Electrical cardioversion was performed in 115 patients: 42 cardioversions in patients taking dabigatran, 33 in patients taking rivaroxaban, and 40 in patients taking warfarin. Table 1 presents the baseline characteristics of the study groups. The mean age was 58.4, 55.7, and 57.6 years in the dabigatran, rivaroxaban, and warfarin groups, respectively (p=0.722, dabigatran vs. warfarin; p=0.462, rivaroxaban vs. warfarin). Male patients comprised 81.0%, 87.9%, and 87.5% of the dabigatran, rivaroxaban, and warfarin groups, respectively (p=0.417, dabigatran vs. warfarin; p=1.000, rivaroxaban vs. warfarin). The mean CHA2DS2-VASc score was 1.2, 0.7, and 1.1 in the dabigatran, rivaroxaban, and warfarin groups (p=0.918, dabigatran vs. warfarin; p=0.078, rivaroxaban vs. warfarin). The concomitant use of aspirin or clopidogrel did not differ between the groups. The median duration of antithrombotic therapy was 28, 30, and 35 days in the dabigatran, rivaroxaban, and warfarin groups, respectively (p<0.001, dabigatran vs. warfarin; p=0.104, rivaroxaban vs. warfarin). The proportion of patients on antithrombotic therapy for ≥3 weeks before electrical cardioversion was 85.7%, 100.0%, and 92.5% in the dabigatran, rivaroxaban, and warfarin groups (p=0.483, dabigatran vs. warfarin; p=0.247, rivaroxaban vs. warfarin).
Transthoracic and TEE Findings
The mean LA diameter in dabigatran, rivaroxaban, and warfarin groups was 46.4, 46.4, and 44.9 mm, respectively (p=0.268, dabigatran vs. warfarin; p=0.305, rivaroxaban vs. warfarin, Table 2). TEE revealed no difference in the incidence of LA SEC between groups (28.6%, 21.2%, and 22.5% in the dabigatran, rivaroxaban, and warfarin groups, respectively; p=0.529, dabigatran vs. warfarin; p=0.895, rivaroxaban vs. warfarin). No thrombi were noted.
Clinical outcomes
All patients were monitored for the first 30 days after electrical cardioversion. No stroke or systemic embolism occurred during the 30-day follow-up period. No patients were found to have major bleeding events.
Subgroup analysis in patients with CHA2DS2-VASc scores ≥2
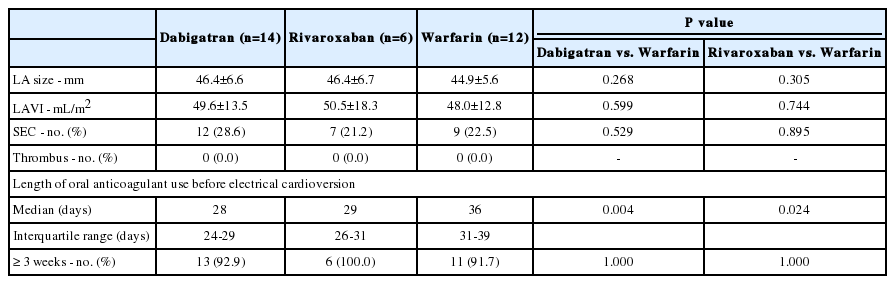
Our study patients had relatively low CHA2DS2-VASc scores, and therefore we conducted a subgroup analysis in patients with CHA2DS2-VASc scores ≥2. No difference in the incidence of LA SEC was found between the groups (Table 3). However, the median duration of antithrombotic therapy was shorter in the dabigatran and rivaroxaban groups than in the warfarin group (p=0.004, dabigatran vs. warfarin; p=0.024, rivaroxaban vs. warfarin).
Discussion
Current guidelines recommend therapeutic anticoagulation with warfarin for 3 weeks before and 4 weeks after electrical cardioversion [8,9]. However, in real-world practice, it often takes longer than 3 weeks to reach therapeutic anticoagulation before electrical cardioversion. One study reported that the median time required to fulfill the anticoagulation recommendations was 5 weeks, and the time from warfarin initiation to actual cardioversion of atrial fibrillation was almost 2 months due to difficulties in INR status and scheduling [13]. To shorten the time to electrical cardioversion, a TEE-guided cardioversion strategy could be used. However, although no LA thrombi were seen on TEE, therapeutic anticoagulation was needed during the pericardioversion and postcardioversion periods [14].
Dabigatran is an oral direct thrombin inhibitor, and its peak plasma concentrations are reached approximately 2 hours after oral administration [15]. The serum half-life of dabigatran is 12 to 17 hours, and patients taking this drug do not require regular monitoring [15]. Rivaroxaban is an oral direct factor Xa inhibitor, and its peak plasma concentrations are reached 3 to 4 hours after oral administration [16]. Therefore, if NOAC therapy is used, only 3 weeks of anticoagulation are needed before electrical cardioversion. Some large-scale studies have reported on the use of NOACs before electrical cardioversion [10-12]. However, these studies were not designed to evaluate the efficacy of short-term NOAC therapy for electrical cardioversion. One retrospective cohort study using short-term dabigatran or rivaroxaban therapy for electrical cardioversion in patients with atrial fibrillation [17] included 53 patients (30 patients with dabigatran and 23 patients with rivaroxaban); 11 of these patients underwent TEE before electrical cardioversion and no thrombi were detected, but SEC data were not reported. A randomized, prospective study exploring the efficacy and safety of once-daily rivaroxaban versus dose-adjusted vitamin K antagonist (VKA) treatment was recently reported [18]. This study showed that the thromboembolic and bleeding risks of rivaroxaban for electrical cardioversion were similar to those observed with VKA treatment; however, the incidence of SEC and differences between groups were not reported.
In our study, no stroke or systemic embolism occurred in any of the patient groups during the follow-up period, and there were no major bleeding events. Although our follow-up period was relatively short, it was sufficient to evaluate thromboembolic events after electrical cardioversion, because most thromboembolic events develop during the first week after electrical cardioversion [7]. In the present study, TEE was performed before electrical cardioversion in all patients. No thrombi were detected, and no differences in the incidence of SEC were found between groups. The incidence of SEC in our study was comparable to that of a previous study [10]. In patients with a CHA2DS2-VASc score ≥2, the incidence of SEC was also not different between groups. These findings support the feasibility of short-term NOAC therapy for electrical cardioversion in patients with atrial fibrillation.
Limitations
Our study has several limitations. First, it was a retrospective observational study. Second, our study patients had lower CHADS2 and CHA2DS2-VASc scores than those of a previous large-scale post-hoc analysis of patients who underwent cardioversion. Therefore, our findings should be interpreted with caution. Finally, because our study had a small sample size, its statistical power to compare rare endpoints is low.
Conclusion
The short-term use of dabigatran or rivaroxaban can be considered safe in patients undergoing electrical cardioversion for atrial fibrillation.
Notes
Disclosures: Drs. Kyoung-Min Park, Seung-Jung Park, June Soo Kim, and Young Keun On receive speaking fees from Boehringer Ingelheim (Germany) and Bayer HealthCare Pharmaceuticals (Germany)