Management of Aborted Sudden Cardiac Arrest with J Wave Syndrome
Article information
Abstract
We report the case of a 19-year-old male who successfully recovered from sudden cardiac arrest. Careful evaluation did not reveal any electrical or structural abnormalities. He underwent implantable cardioverter defibrillator (ICD) implantation, with a diagnosis of idiopathic ventricular fibrillation (VF). Three months later, VF recurred and was successfully terminated by ICD shock. Electrocardiogram (ECG) revealed a slurred type J point elevation at the inferolateral leads with a horizontal/descending ST segment change, which was not present during the initial hospitalization. Cilostazol was prescribed to prevent further lethal ventricular arrhythmias. Subsequently, no arrhythmic events were reported, and the J wave disappeared at the follow-up ECG. However, recurrent VF episodes with an interval of 1–2 weeks occurred 1 year later, and were terminated by ICD shock. Simultaneous ECG revealed a J point elevation at the inferolateral leads. Cilostazol was replaced by quinidine. Subsequently, no arrhythmic event recurred for over 12 months. Serial follow-up ECG is needed to identify masked inherited primary arrhythmic syndromes in sudden cardiac arrest survivors diagnosed with idiopathic VF. Cilostazol and quinidine might be good therapeutic options to prevent further lethal events in cases where the J wave syndrome is present with recurrent ventricular arrhythmias.
Introduction
Lethal ventricular arrhythmias can occur even in the absence of structural heart disease. Inherited primary arrhythmia syndrome (IPAS) is a rare condition, but is often associated with high mortality rates. Despite meticulous evaluation, the underlying cause of sudden cardiac arrest (SCA) remains idiopathic in a majority of survivors. IPAS is often revealed during serial follow-up examinations because electrocardiographic abnormalities of IPAS tend to change spontaneously. The J wave syndrome is a recently discovered form of IPAS and is an important cause of SCA in relatively young patients. We report the case of a 19-year-old male who survived SCA and a diagnosis of J wave syndrome was made subsequently.
Case
A 19-year-old male was transferred to the emergency department with aborted sudden cardiac arrest. He had no previous history of hypertension, diabetes mellitus, dyslipidemia, or other cardiac problems. He was a student with ordinary activity levels. Cardiac arrest developed during sleep. When the emergency team arrived, an automated external defibrillator demonstrated ventricular fibrillation (VF), which was terminated by three rounds of defibrillation. After the successful recovery of spontaneous circulation, the patient underwent hypothermia treatment. Subsequently, careful evaluation was performed to identify the cause of sudden cardiac arrest. None of the examinations performed, including electrocardiogram (ECG), holter monitoring, coronary angiogram, brain computer tomography, and flecainide or epinephrine drug challenge test, revealed any abnormalities. Electrophysiological studies did not induce any ventricular tachycardia. Finally, a diagnosis of idiopathic VF was made and the patient underwent implantable cardioverter-defibrillator (ICD) implantation to protect him from further lethal ventricular arrhythmias.
The patient had an uneventful recovery after being discharged from the hospital. However, 3 months later, an ICD shock occurred, which was revealed to be a VF event at ICD interrogation (Figure 1). At that time, a simultaneous ECG demonstrated the presence of a newly developed J point elevation at the inferolateral lead, which was of the slurred type with a horizontal/descending ST segment change (Figure 2). Cilostazol was prescribed to prevent further lethal ventricular arrhythmias. Nevertheless, recurrent VF episodes with an interval of 1 to 2 weeks occurred 1 year later, and were terminated by ICD shock (Figure 3). At that time, ECG also demonstrated a J point elevation at the inferolateral lead, which was not apparent during the ‘no-event’ period. To minimize shock therapy, cilostazol was switched with quinidine, as class Ic anti-arrhythmic agents directly inhibit Ito, thereby decreasing the J point elevation resulting in decreased the transmural dispersion of repolarization and refractoriness. No arrhythmic events were reported subsequently and ECG revealed no J wave.
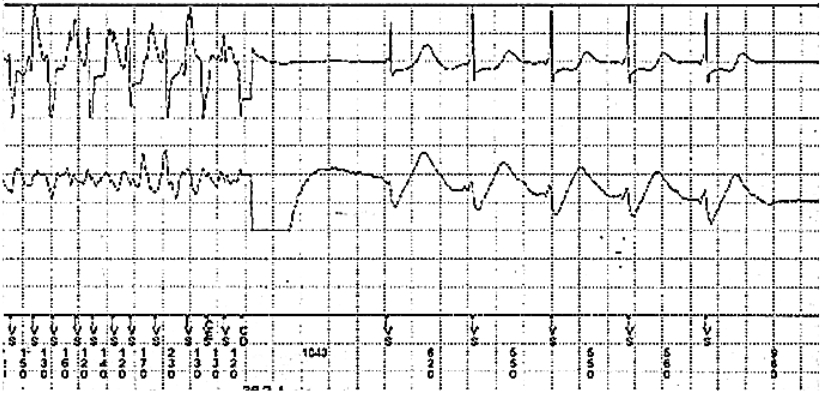
Interrogated intracardiac electrogram from an implantable cardioverter defibrillator (ICD). Ventricular fibrillation was present and was terminated by ICD shock 36 J.
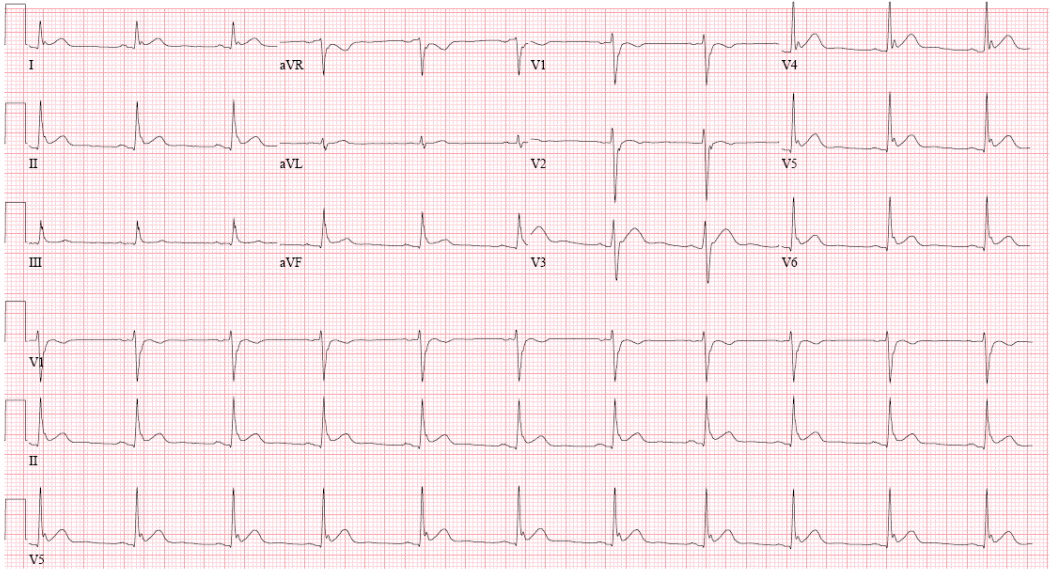
Electrocardiogram (ECG) after successful defibrillation from ventricular fibrillation. A newly developed J point elevation at the inferolateral lead of the slurred type with a horizontal/descending ST segment change was detected by ECG.
Discussion
The early repolarization pattern in ECG, known as the J wave, is considered to be benign. Recent studies, however, have reported that the J wave may be associated with an increased risk of death [1,2]. Additionally, early repolarization has been reported to be associated with recurrent VF in cardiac arrest survivors [3]. Rosso et al. have termed early repolarization with a horizontal/descending ST segment as “malignant”, because it was more strongly correlated with idiopathic ventricular fibrillation among all types of early repolarization [4]. In the present case report, a horizontal/descending type of early repolarization pattern was documented in the ECG. This abnormal pattern, which exhibited dynamic changes with time, might cause recurrent VF episodes.
The J wave is formed due to an outward shift in the balance of the membrane ionic currents at the end of the phase 1 and phase 2 action potentials. In these phases, an outward current occurs mainly due to the activation of the transient outward current (Ito), while the inward current is a result of the activation of an inward calcium current (ICa) and an inactivating component of an inward sodium current (INa). The loss of the action potential dome in the epicardium, but not in the endocardium, results in the development of a marked transmural dispersion of repolarization and refractoriness. This event is responsible for the development of a vulnerable window during which a premature impulse or extrasystole can induce a reentrant arrhythmia [5-7].
Although the primary therapy for VF is ICD implantation, shock reduction and the prevention of recurrent ventricular arrhythmia should be considered. A pharmacologic intervention, which either decreases the membrane outward currents, or increases the inward currents, prevents the loss of the action potential dome in the right ventricular epicardium. Q uinidine has been shown to directly inhibit the Ito in addition to a secondary inhibition due to an increase in the heart rate by a vagolytic action [5,7]. Experimental studies have shown quinidine to be effective in restoring the epicardial action potential dome, thus normalizing the ST-segment and preventing phase 2 reentry and polymorphic VT [8]. Cilostazol, an oral phosphodiesterase type-III inhibitor has been shown to increase the ICa due to the inhibition of the phosphodiesterase activity in the ventricular myocytes and decreases the Ito due to an increased heart rate which again is secondary to an increased ICa in the sinus node [9].
In the present case report, the patient underwent ICD implantation with the initial diagnosis of idiopathic VF. However, J point elevation occurred during late follow-up, which was related with recurrent ventricular arrhythmia, and potential preventive strategies were indicated. Pharmacological therapy with cilostazol and quinidine successfully suppressed lethal ventricular arrhythmias. Therefore, careful and serial follow-up evaluation is essential in patients with idiopathic VF because unrevealed IPAS might be present. Additional therapeutic strategies may be considered to prevent and minimize the occurrence of further lethal arrhythmias.
