Design of Korean Noninvasive Risk Evaluation Study for Sudden Cardiac Death from Infarction or Heart Failure – Myocardial infarction study of K-REDEFINE registry -
Article information
Abstract
Background and Objectives
Despite significant advances in the treatment of acute myocardial infarction (MI), the prevention of sudden cardiac death (SCD), the most common mode of death in patients with MI, remains challenging. Furthermore, previous Korean MI registries did not address the issue of post-MI SCD. Additional risk stratifiers of post-MI SCD are still required to compensate for the limitation of using left ventricular ejection fraction to predict lethal arrhythmic events.
Subjects and Methods
We designed the first Korean prospective nationwide multicenter registry primarily focused on SCD; the Korean noninvasive Risk Evaluation study for sudden cardiac DEath From INfarction or heart failurE (K-REDEFINE). The registry consists of 2 groups of patients presenting with (1) acute MI or (2) acute heart failure (HF) at 25 tertiary referral cardiovascular centers. The primary endpoint of the MI group study of K-REDEFINE registry is the incidence and risk factors of post-MI SCD. In particular, the association between the risk of SCD and non-invasive Holter-based electrocardiogram (ECG) variables will be evaluated, such as T-wave alternans (marker of repolarization heterogeneity) and heart rate tur-bulence/variability (a marker of autonomic function). Other secondary study outcomes include atrioventricular arrhythmias, HF-related admission, repeated myocardial ischemic events, stroke, and overall deaths.
Conclusion and Perspective
The K-REDEFINE registry will provide new prospects for the better management of MI patients with high risk of SCD by clarifying the burden and predictors of SCD and the clinical utility of various non-invasive ambulatory ECG-based variables in risk stratification for SCD in this patient population.
Introduction
Despite recent significant advances in the treatment of acute myocardial infarction (MI), sudden cardiac death (SCD) continues to be the most common cause of death after acute MI, acomprising 30–40% of overall mortality according to Western studies.1,2 The incidence of acute MI in Korea has also increased by around 10% each year during the last several decades,3 with the 1-year mortality (11%) comparable to those from many developed countries due to westernization of lifestyles and a rapidly aging population.4 Moreover, with the advent of potentially life-saving therapy such as implantable cardioverter-defibrillator (ICD), a more accurate risk stratification of patients post-MI becomes more critical in clinical practice.
Left ventricular (LV) ejection fraction (EF) has been one of the most widely used criteria for determining ICD implantation as a primary prevention strategy against post-MI SCD.5–7 However, more than half of SCD cases developed in MI survivors with a LV EF>40%, showing a low positive predictive value of LV EF.8 Additionally, in a recent study that enrolled post-MI patients with a reduced LV EF (≤40%), lethal ventricular tachyarrhythmia was documented in only 8.0% over a 2-year follow-up period.9 Prophylactic ICD use after acute MI failed to show mortality benefit when used in the first month during which the risk of SCD was reportedly highest.10
Given the limitation of LV EF as a risk factor, continuous efforts to find another risk factor of SCD in patients with acute MI are required.11 T-wave alternans (TWA) and heart rate turbulence (HRT) are non-invasive surrogate markers of cardiac electrical instability and autonomic dysfunction, respectively. A close association between the abnormal values of TWA and/or HRT and an increased risk of SCD has been suggested by many clinical studies.12–15 However, the predictive values of TWA and HRT for SCD have never been evaluated in Korean patients following acute MI.
Therefore, we aimed to assess the incidence and risk factors of SCD in patients with acute MI using a newly designed nationwide multicenter MI registry, and evaluate the efficacy of TWA and HRT alone or in combination with other parameters for predicting SCD in this patient population.
Subjects and Methods
Study population
The K-REDEFINE (Korean noninvasive Risk Evaluation study for sudden cardiac DEath From INfarction or heart failure) is designed as a prospective nationwide multicenter registry encompassing 2 groups of patients presenting with (1) acute MI or (2) acute HF at 25 tertiary referral cardiovascular centers in South Korea. Enrollment of patients began in September 2015 and is expected to be completed by the first half of 2018. Follow-up is planned for up to 5 years after finishing the enrollment.
Patients who show evidence of myocardial necrosis as suggested by a rise and/or fall of cardiac enzyme (preferably troponin) with at least one of the following will be screened for enrollment eligibility into the acute MI study of the registry (i) symptom of myocardial ischemia, (ii) new-onset significant change in ST-segment/T-wave, (iii) new-onset left bundle branch block, (iv) development of pathological Q-wave on the ECG, (v) new loss of viable myocardium or new regional wall motion abnormality on imaging studies, or (vi) intracoronary thrombus identified on coronary angiography. Patient aged 19 years or more admitted for new-onset acute MI and showing sinus rhythm (SR) will be included in this study. However, patients with (1) persistent/permanent atrial fibrillation (AF), (2) ventricular paced rhythm, (3) life-threatening co-morbidity with life expectancy <1 year, (4) end stage renal disease requiring renal replacement therapy, or (5) inability or unwillingness to provide consent will be excluded. The patients will be categorized into ST-segment elevation MI (STEMI) or non-STEMI by the attending physician. The extent of coronary disease will be decided by the number of major epicardial coronary arteries with significant stenosis (>50%).
Regarding the estimation of the sample size for the present study, we decided to place no limitation on it, because there has been no registry primarily dealing with SCD in Korean patients with MI or HF. Then, it was calculated based upon the number of patients enrolled in the previous registries established in Korea16–19; 1000 consecutive patients will be enrolled for each acute MI and HF group respectively, with a total of 2,000 patients.
All enrolled patients will provide written informed consent. Obtaining an informed consent from legal representatives will be permitted when it is impossible for the patients to provide consent by themselves due to disability. The study protocol was approved by the local institutional review board of each participating center.
Study protocol and data collection
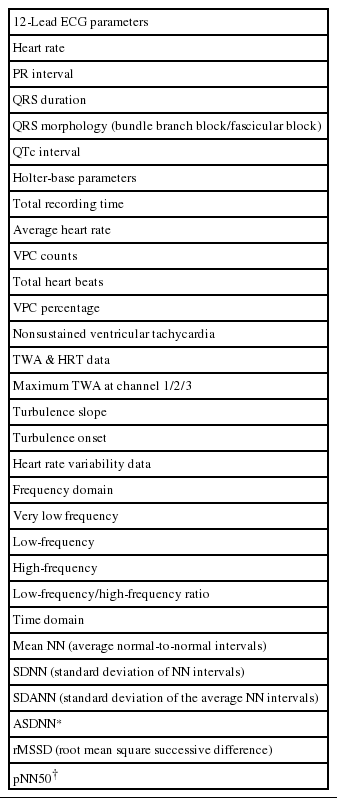
The study is funded by a grant from the Korean Heart Rhythm Society (KHRS). The Non-Invasive Study Steering Committee of the KHRS designed the study protocol and case report forms for the present study. A web-based electronic case report form (e-CRF) will be used for prospective data collection by the attending physicians at each participating center with the assistance of a clinical research coordinator. The e-CRF for the K-REDEFINE registry was made with support from GE Healthcare Korea. However, no access to patient-related data will be allowed by the sponsor. Direct participant identifiers including names, personal identification numbers, and medical record numbers will be substituted with linking codes. Specific baseline parameters such as demographics, clinical characteristics, surface 12-lead, and ambulatory ECG data, including TWA and HRT values, echocardiographic variables, and laboratory results, will be obtained during the index admission. Table 1 shows details of the baseline clinical data that will be collected.
Measurement of TWA and HRT using ambulatory ECG recording
TWA refers to the beat-to-beat oscillation in the shape and amplitude of the ST-segment and/or T-wave (Figure 1). This alternating phenomenon has been proven to be closely related to the temporospatial heterogeneity of repolarization in many in vitro and in vivo experiments.12 In general, the greater the magnitude of TWA, the higher the risk of arrhythmogenesis.20–22 Ischemia-induced intercellular electrical decoupling and intracellular defects involving calcium handling are implicated in the development of concordant/discordant alternans followed by heterogeneous conduction block and re-entry.
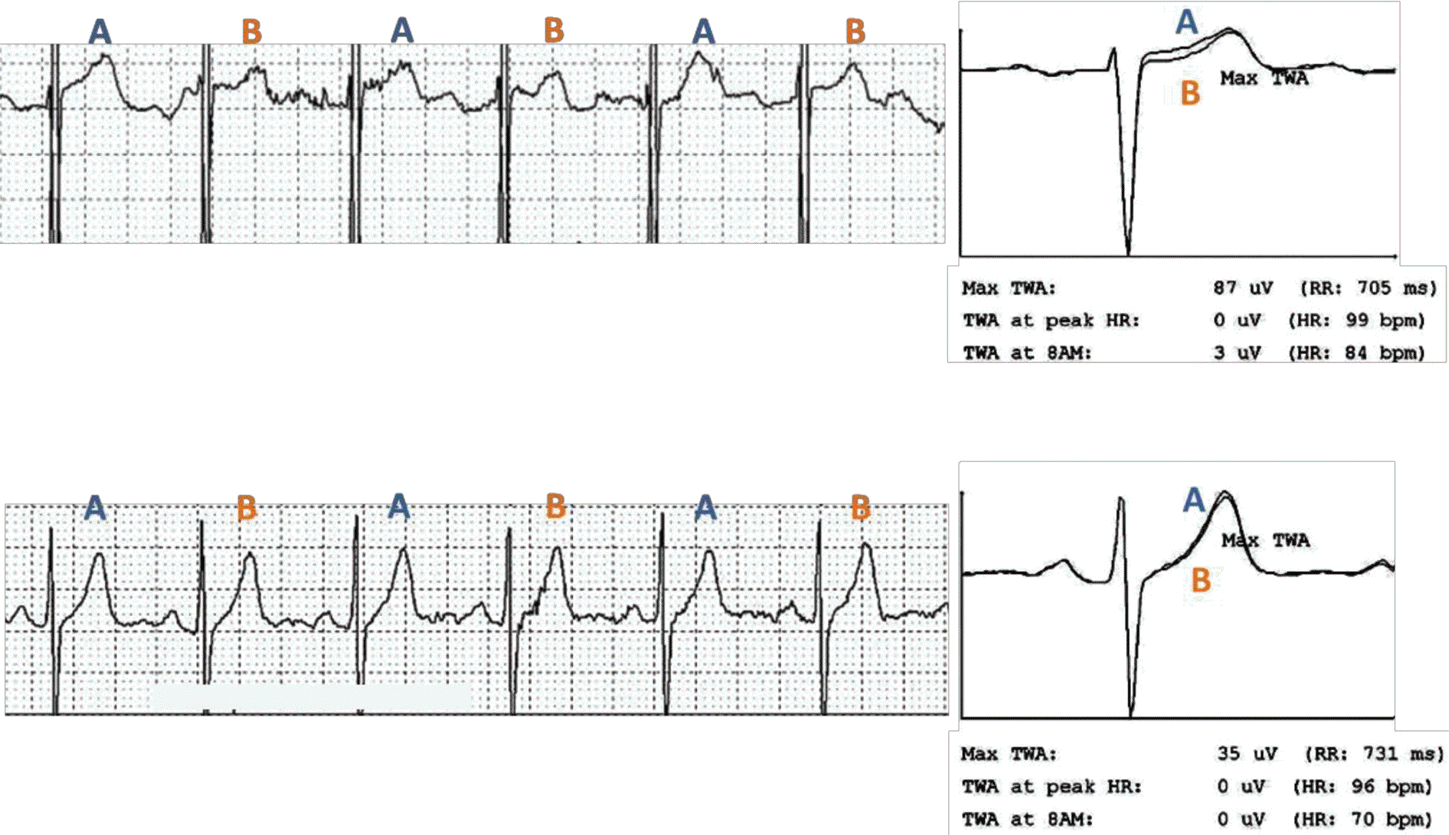
Measurement of T-wave alternans using moving average method bpm, beats per minute; HR, heart rate; TWA, T wave alternans
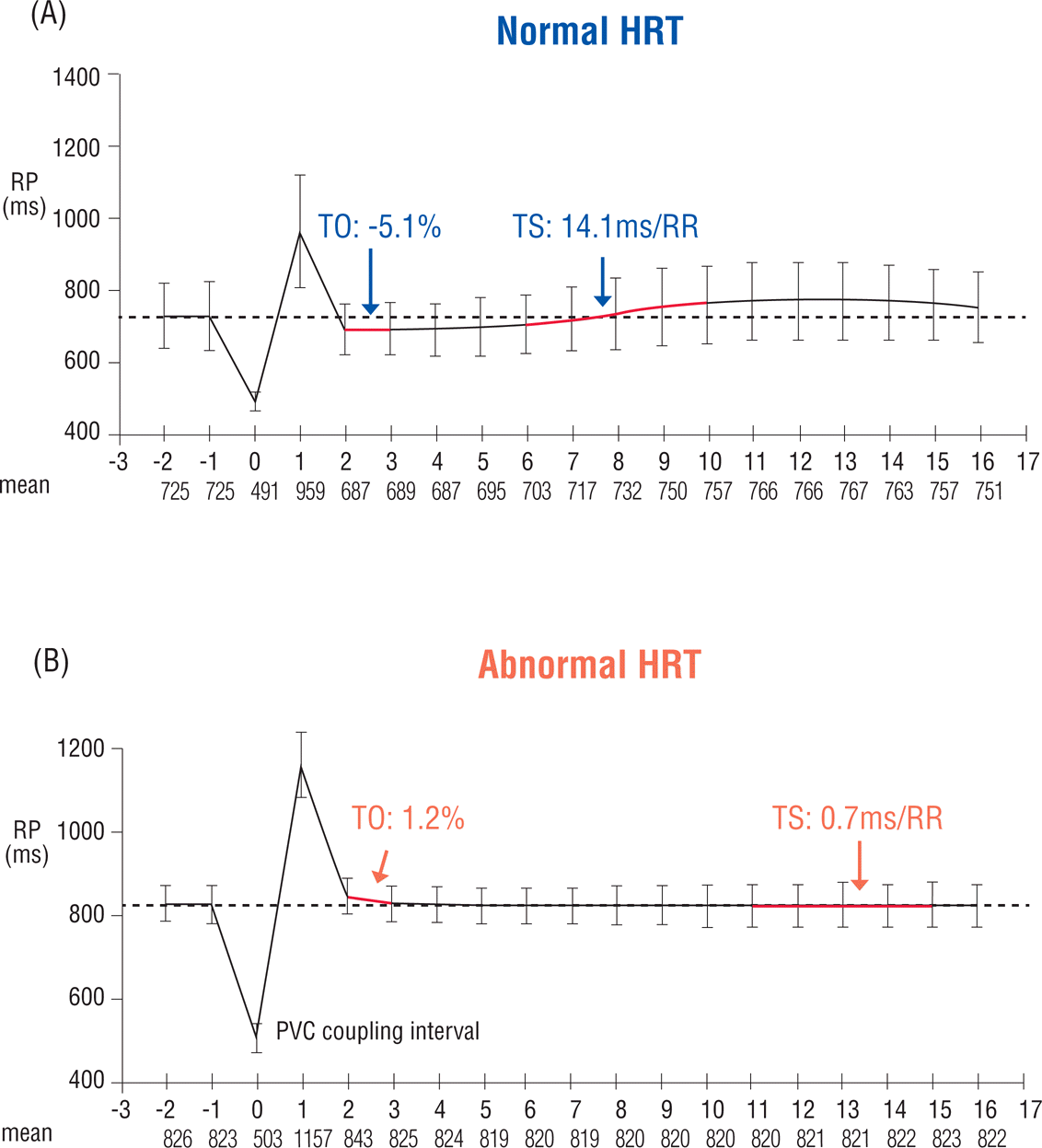
HRT refers to a biphasic change in HR after ventricular premature contractions (VPCs) with initial acceleration and subsequent deceleration (Figure 2). The initial HR acceleration is caused by transient vagal withdrawal due to VPC-induced blood pressure (BP) lowering, whereas the subsequent HR deceleration is related to baroreflex-mediated transient BP elevation. Therefore, HRT assessment is an indirect assessment of baroreflex function and patients with structural heart disease such as MI and/or ischemic cardiomyopathy frequently show a blunted HRT response.13,22,23
In the present study, TWA and HRT will be prospectively measured using ambulatory ECG data in patients with acute MI. Holter electrocardiograms will be recorded during the index admission or within 3 months after discharge. An ambulatory 3-lead SEER Light Digital Holter monitor (GE Healthcare Inc., Milwaukee, WI, USA) will record ECG data at a sampling rate of 125 samples per second. All participating centers will be encouraged to record Holter ECG data for at least 20 hours to incorporate overall circadian variations in the TWA and HRT levels during daytime and nighttime. Before lead attachment, a careful skin preparation will be performed to ensure a high-quality signal and minimize noise.12 The core laboratory (Samsung Medical Center) will collect raw ambulatory ECG data recorded at each center, and analyze them with standardized analysis settings. Details are provided in Table 2. A MARS 8000 Holter analyzer (GE Healthcare Inc., Milwaukee, WI, USA) will be used for the analysis of TWA and HRT values. Three precordial leads (V1, V3, and V5) will record the electrocardiograms; the TWA value in each lead will be calculated using the modified moving average method (MMA) based on the time-domain algorithm of TWA shown in Figure 1.11,13 Unlike the conventional spectral method that provides qualitative data only on TWA level, the MMA method provides quantitative assessment of the TWA.12 After the initial measurement of TWA, review and confirmatory processes will be followed. During the process, 2 dedicated cardiologists with the assistance of 1 ECG specialist will examine the presence of significant noise or artifacts on the ECG recordings, and select the maximum TWA value obtained in the ECG rhythm strip without significant noise or artifacts as a final value. For HRT assessment, the following 2 quantitative values will be measured: the turbulence onset (TO) and slope (TS), as shown in Figure 2. TO is calculated using the following formula:
[%], where RR-2 and RR-1 are the sinus RR intervals recorded immediately before the VPC coupling interval, and RR1 and RR2 are the sinus RR intervals recorded immediately following the compensatory pause. TS is measured as the maximum positive regression slope obtained over any 5 consecutive sinus RR intervals within the first 15 sinus rhythm RR intervals following the VPC.13 Values of TO >0% and TS <2.5 ms/RR interval will be defined as abnormal, as reported in the previous studies.13 HRT will be considered as abnormal when either the TO or TS values are abnormal. In addition, patients will be categorized into 3 groups as follows: HRT0 (both TO and TS normal), HRT1 (either TO or TS abnormal), and HRT2 (both TO and TS abnormal). In addition to TWA and HRT measurements, other Holter-based variables will be collected using the same system, including time- and frequency-domain HR variability (HRV) parameters. The details of Holter-based variables analyzed in the present study are presented in Table 3.
Discharge information and clinical outcomes during follow-up
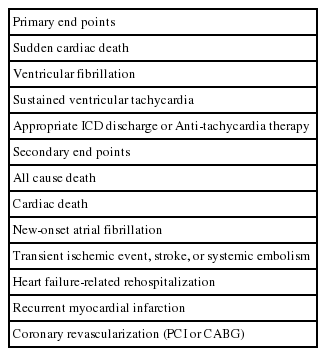
Information on the status at discharge and during follow-up will be gathered using standardized report forms. Discharge information will incorporate survival status, modes of death (non-cardiac, non-sudden cardiac, or SCD) if death occurred, revascularization modalities (percutaneous intervention, bypass surgery, or medical treatment alone), types of cardiac device therapy (pacemaker, defibrillator, or biventricular pacemaker) if performed, and duration of hospitalization. Follow-up information will be collected at unplanned visits associated with study endpoints and predefined schedules, corresponding to 1, 3, 6, and 12 months after discharge and annually thereafter up to 5 years. The primary endpoint is SCD, which is defined as unexpected death due to cardiac causes that occurs in a short time period (within 1 hour of symptom onset or unwitnessed death during sleep). Other primary and secondary outcomes are shown in Table 4. An independent Clinical Event Adjudication Committee consisting of experts in the management of patients with MI and HF will verify all clinical events such as death and re-hospitalization. The survival status and outcome data for subjects lost to follow-up will be supplemented by telephone interview in which a structured questionnaire will be used in all participating centers.
Discussion
Burden of sudden cardiac death in Korea
The annual incidence of SCD in Korea was estimated to be 40–45 per 100,000 persons according to a complete enumeration survey performed every year from 2006 by the Korean Centers for Disease Control & Prevention using medical records of emergency medical service (EMS) including out-of-hospital SCD information;24,25 annually, more than 30,000 persons have experienced out-of-hospital SCD since 2009, which is similar to those from other developed countries such as the USA, Canada, Ireland, and Japan. Moreover, the annual incidence of SCD (40–45 per 100,000 persons) was likely underestimated, because the in-hospital SCD cases were not included in the survey. However, regrettably, the overall survival-to-discharge rates in Korea (3.0%–4.0%) were much lower than those of other studies carried out in North America, Europe, and Japan (8%–14%) during the same 24,25
Need for the K-REDEFINE registry
A significant portion of Korean patients with MI have probably experienced SCD similar to those in other developed countries because westernized lifestyles are becoming more prevalent in Korea. Despite the clinical importance of SCD in the population with MI, previous multicenter Korean MI registries, although nationwide and well-designed, have failed to provide enough data associated with SCD;18,19 the Korea Acute Myocardial Infarction Registry (KAMIR) and the Korea Working Group on Myocardial Infarction (KorMI) registries incorporating more than 34,000 patients with acute MI have provided considerable valuable insights on many aspects of acute MI. However, the KAMIR and KorMI registries did not collect data associated with SCD. Information on anti-arrhythmic medications, the use of a cardiac implantable electronic device, and ventricular arrhythmic events are also lacking.
Furthermore, this lack of data on post-MI SCD in Korea might lead to underutilization of potentially life-saving therapies such as ICD or cardiac resynchronization therapy (CRT) devices. Indeed, the rate of ICD/CRT-defibrillator (CRT-D) implantation was below 1.5% according to a recent registry study conducted in Korean patients with HF, another high-risk group for SCD.26 Additionally, according to the 11th world survey of cardiac pacing and ICD, the annual rate of new ICD or CRT-D implantation in Korea in 2009 (6 per million) was much lower than that in Japan (42 per million) or in the United States of America (434 per million), even if corrected by population.27
Given this situation, the K-REDEFINE registry will provide clinicians with valuable assistance in the identification of SCD predictors, and the adequate and timely allocation of potentially life-saving therapies to those at with MI at high risk for SCD. Indeed, another study using Korean EMS data demonstrated that witnessed collapse and a pre-hospital shockable rhythm were identified as independent predictive factors for survival-to-discharge. Therefore, the adequate use of ICD/CRT-D therapy might improve outcomes in Korean patients with MI.28
TWA and HRT for SCD
Abnormal TWA and HRT, as surrogate markers of repolarization abnormality and cardiac autonomic dysfunction respectively, are closely associated with an increased risk of SCD. Therefore, they have already been investigated in many studies. However, TWA was assessed only qualitatively using a spectral method in most studies. In addition, several studies where TWA was measured quantitatively using Holter data had a limitation of retrospective analysis or small-sized nested case-control design.12,20,21 Regarding HRT, most post-MI HRT studies performed retrospective analyses and used total or cardiac death as the primary endpoint instead of SCD.13,22,23
The present K-REDEFINE study will be one of the largest cohorts to evaluate prospectively the association between TWA/ HRT and SCD in patients with MI using ambulatory ECG data. Additionally, TWA will be measured quantitatively using the MMA method. Therefore, the K-REDEFINE study could enhance our understanding of the role of TWA and HRT in identifying patients with MI at high risk of SCD.
Conclusion
The MI study of the K-REDEFINE registry is the first Korean prospective study primarily focused on SCD in patients with MI from 25 centers in Korea. The K-REDEFINE registry will pioneer better management of patients with MI at high risk of SCD by elucidating the burden and risk factors of SCD and the clinical utility of various non-invasive ambulatory ECG-based parameters in risk stratification for SCD in this patient population.
Acknowledgements
The present study was supported by the KHRS. We express our sincere gratitude to the investigators at the 25 participating university hospitals/medical centers for their contributions to this study. The participating centers are as follows in alphabetical order: Ajou University Hospital, Asan Medical Center, Chonnam National University Hospital, Chosun University Hospital, Chung-Ang University Hospital, Chungbuk National University Hospital, Chungnam National University Hospital, Daegu Catholic University Medical Center, Gachon University Gil Medical Center, Inha University Hospital, Inje University Busan Paik Hospital, Inje University Ilsan Paik Hospital, International St. Mary's Hospital, Jeju National University Hospital, Keimyung University Dongsan Medical Center, Korea University Medical Center, Korea University Ansan Hospital, Kosin University Gospel Hospital, Samsung Medical Center, Sejong General Hospital, Severance Hospital of Yonsei University College of Medicine, Wonkwang University Hospital, Wonju Severance Christian Hospital, and Yeungnam University Hospital.