The Impact of the CHA2 DS2-VASc Score on Recurrence of Atrial Fibrillation after a Single Catheter Ablation and Atrial Remodeling in Patients with Non-Valvular Atrial Fibrillation
Article information
Abstract
Background and Objectives
This study aimed to evaluate the impact of the CHA2 DS2-VASc score on atrial fibrillation (AF) recurrence after a single catheter ablation procedure in patients with non-valvular AF. We also investigated the correlation between CHA2 DS2-VASc score and atrial substrate.
Subjects and Methods
This study evaluated 151 patients who underwent catheter ablation of non-valvular AF. The study population was stratified into group 1 (<2, n=72) and group 2 (≥2, n=79) by CHA2 DS2-VASc score. The CHA2 DS2-VASc score was analyzed as a continuous and categorical value for evaluating its impact on AF recurrence after catheter ablation. The left atrial voltage data were analyzed by the categorical values of this score.
Results
Post-ablation recurrence (31.6% vs. 18.1%, p=0.046) was observed more frequently in group 2. The mean area of the low-voltage zone was 75.64±24.81 cm2 and 94.44±28.09 cm2 in groups 1 and 2, respectively (p=0.005). The left atrial mean voltage in group 2 was 0.99±0.31 mV, significantly lower than that (1.49±0.67 mV, p=0.001) in group 1. The CHA2 DS2-VASc score was the independent predictor with a modest predictive value for AF recurrence after catheter ablation.
Conclusion
Our study showed that CHA2 DS2-VASc score was associated with atrial remodeling and could be useful in stratifying post-ablation recurrence in patients with non-valvular AF.
Introduction
Atrial fibrillation (AF) is an electric disorder of the heart but it may more frequently be the result of structural cardiac and systemic diseases than present an isolated disorder. The comorbidities related to AF and each patient's status can be a determining factor of prognosis and related complications. For this reason, the CHA2 DS2-VASc score is commonly used to predict the annual risk of embolic stroke in patients with non-valvular AF.1
Catheter ablation has been a cornerstone of rhythm control for AF for the past two decades. However, it is well known that the recurrence rate after a single catheter ablation procedure exceeds 40%.2 Many studies have demonstrated predictors of worse outcomes after catheter ablation such as an increased size and fibrosis of the left atrium (LA), AF classification and duration, hypertension, and elevated inflammatory and oxidative marker levels.1–4 However, the prognosis after catheter ablation of AF may not be determined by a sole predictor. Therefore, the main purpose of our study was to evaluate the prognostic impact of the CHA2 DS2-VASc score on rhythm outcomes after a single catheter ablation procedure in patients with non-valvular AF. We also investigated the correlation between CHA2 DS2-VASc score and atrial substrate.
Subjects and Methods
Patients
The present study enrolled 151 consecutive patients with symptomatic drug-refractory AF who underwent catheter ablation between March 2010 and February 2013. Patients with permanent AF, prior AF ablation, congenital cardiac anomalies or valvular heart disease, malignancies, intolerance to oral anticoagulation, alcohol abuse, an LA volume index ≥60 mL/m2 on transthoracic echocardiography at baseline, and intracardiac thrombi documented by transesophageal echocardiography were excluded from the study. Each patient's CHA2 DS2-VASc score was calculated during the index hospitalization by assigning 1 point each for age 65–74 years, hypertension, diabetes mellitus (DM), congestive heart failure, and vascular disease; in female patients, 2 points were assigned for age ≥75 years and a previous history of stroke or transient ischemic attack.1 By CHA2 DS2-VASc score, the study population was stratified into groups 1 (<2, n=72) and 2 (≥2, n=79). AF was classified as paroxysmal or non-paroxysmal AF (PAF) (persistent and long-standing persistent AF) according to the current guidelines.1 The study protocol was approved by the institutional review board at Yeungnam University Hospital. All patients provided informed consent.
Measurement of left atrium size and volume
We measured the LA size and volume of all patients before the catheter ablation. On transthoracic echocardiography, the LA size was measured by the anteroposterior dimension in the parasternal long-axis view. The LA volume was calculated using the prolate ellipsoid method.5 The LA volume index was represented as the LA volume divided by body surface area (mL/m2). LA volume was calculated automatically with computed tomography (CT) using the built-in software (Aquaris iNtuition, TeraRecon Inc., San Mateo, CA, USA) and was recorded after removal of the pulmonary veins (PVs) from each ostium.
Ablation procedure and outcomes
Class Ic antiarrhythmic drugs were discontinued prior to at least five half-lives and amiodarone was stopped at least a month before the ablation. Patients underwent an electrophysiological study and catheter ablation under conscious sedation. We used a three-dimensional mapping system (NavX™; St. Jude Medical, St. Paul, MN, USA) in all patients with pulmonary venography to identify the anatomical position of each PV ostium. The three-dimensional LA geometry was merged with the CT image to create a detailed reconstruction. We used a 7F ablation catheter with a 3.5-mm irrigated tip (Cool Flex™ or Cool Path™ Duo irrigated catheter; St. Jude Medical, St. Paul, MN, USA) and the power was titrated to 25–35 W with the temperature set to <40°C. The PV isolation was performed with continuous circumferential lesions by encircling both PV ostia including the carinas in all patients. The ablation was performed at the atrial side at least 0.5–1 cm away from the PV ostia. Successful PV isolation was confirmed by the dissociation or absence of PV potentials. Furthermore, we confirmed the entrance and exit blocks using a ring catheter during sinus rhythm. In the case of PAF, we tried to find and ablate any non-PV foci that were related to the initiation of AF. To find the non-PV foci, isoproterenol (10 µg/kg/minute) or adenosine (12–20 mg bolus) was infused. If there was no spontaneous beat, we performed short bursts of rapid pacing from the right atrium or coronary sinus. In cases of non-PAF, our routine lesion set included linear ablation at the anterior roof and mitral isthmus. If the AF was terminated during the linear lesion formation, the ablation application was stopped. After that, an induction test was performed with rapid atrial pacing from the right atrium and coronary sinus until a 2:1 atrial capture occurred under an isoproterenol infusion (10–20 µg/kg/ minute). If the AF was not terminated or was induced again, a posterior wall isolation and complex fractionated electrogram (CFE) ablation was attempted. CFE is defined as an atrial electrogram with a very short cycle length (≤120 ms) and/or multiple deflections perturbing the baseline averaged over a 6-second recording period. Each CFE was displayed as a color-coded area by the NavX system. The ablation was completed and the induction test was attempted again if the AF was terminated during the ablation. However, we performed direct current cardioversion to restore sinus rhythm if the AF did not stop or was still inducible.
Acute procedural success was defined as the complete isolation of all PV and non-PV foci as well as bi-directional block on the linear lesions. We analyzed the rate of AF termination by ablation and any atrial tachyarrhythmia induction after ablation. Procedural-related complications, including cardiac tamponade, vascular access–related complications, PV stenosis, atrioesophageal fistula, and procedure-related strokes were also analyzed.
Left atrium voltage mapping
We created sequential contact voltage maps of the LA in 80.8% of our study population except for those patients who underwent a posterior wall box ablation or complications such as cardiac tamponade during the procedure. An ablation catheter was used to obtain roving signals when the ablation catheter contacted the atrial wall during sinus rhythm after the ablation procedure. The signals were obtained from at least 100 sites within the LA; the site had 442 points (minimum, 103; maximum, 1,057 points). The local voltages were measured in each roving signal as the bipolar peak-to-peak voltage and compared to evaluate the differences in the electroanatomical remodeling according to the CHA2 DS2-VASc score. A low-voltage zone (LVZ) was defined as an area with a local voltage value ≤0.5 mV. The LA and LVZ areas were measured using the Field Scaling Function software (EnSite version 8; St. Jude Medical, St. Paul, MN, USA). We used the LVZ/LA area ratio to assess the relative area of the LVZ. Furthermore, we compared the mean peak-to-peak voltage in the LA, LVZ, and non-LVZ areas.
Post-ablation management and follow-up
The use of antiarrhythmic drugs after the procedure was at the operators' discretion but did not exceed the blanking period of 12 weeks. All patients were scheduled for a follow-up visit 1 week after discharge and attended visits every 1 or 2 months until 15 months after the procedure. A standard surface electrocardiogram (ECG) was performed and their symptoms were documented during each follow-up visit. The subjects underwent 24-hour Holter monitoring every 3 months from the end of the blanking period. Patients who reported symptoms suggestive of arrhythmic episodes also underwent symptom-triggered event recording. The study endpoint was set as an AF recurrence during the follow-up period outside the blanking period. According to the current guidelines, AF recurrence was defined as any atrial tachyarrhythmia event including AF, atrial tachycardia, or atrial flutter lasting ≥30 seconds on surface ECG, Holter monitoring, or event recording.4
Statistical analysis
Although the CHA2 DS2-VASc score analyses were performed by category, statistical tests were performed using individual values. The Spearman rank correlation, the Wilcoxon rank-sum test, or the Kruskal-Wallis test was used for continuous or categorical variables, respectively. A Cox proportional hazards model was used to compare the AF recurrence-free survival rate at 1 year after the blanking period. Variables of p<0.05 on univariate Cox analysis were used in the multivariate analysis. A linear regression analysis was conducted to test the multi-collinearity among these variables before the Cox regression analysis. Variables with a variance inflation factor ≥10 were excluded from the Cox regression model. The linear or Cox regression analysis tested the continuous and categorical values of the CHA2 DS2-VASc score with other confounders, respectively. Stepwise elimination and backward selection were used to select the most powerful predictive variables in the Cox regression model. For continuous variables, the Cox model assumption of linearity between the variable and the hazard ratio (HR) of the outcome was assessed by a linear spline and fitting restricted cubic splines. Life-table estimates for post-ablation recurrence were determined and presented as event curves for the two groups defined according to the CHA2 DS2-VASc score. Comparisons between the areas under the receiver operating characteristic (ROC) curves were performed using the Wilcoxon test. All p values were two-sided, and a p value <0.05 was considered to indicate statistical significance. The analyses were performed using IBM SPSS version 19.0 (IBM Co., Armonk, NY, USA).
Results
Baseline characteristics
The mean age and CHA2 DS2-VASc score of our study population were 58.6±8.3 years and 1.8±1.3, respectively. The baseline characteristics by CHA2 DS2-VASc score are shown in Table 1. The mean CHADS2 score in each group was lower than the mean CHA2 DS2-VASc score, and the ratio of the patients with CHADS2 scores ≥2 was 67.1% in group 2. Of the comorbidities belonging to the CHA2DS2-VASc score, the prevalence of hypertension, DM, prior stroke, and peripheral vascular disease were significantly higher in group 2. The size and volume of the LA on the echocardiogram and CT in group 1 were similar to those in group 2. In the chemistry data, an increased high-sensitive C-reactive protein (hs-CRP) was more often seen in group 2. Patients in group 2 had a more decreased glomerular filtration ratio with a higher serum creatinine level.
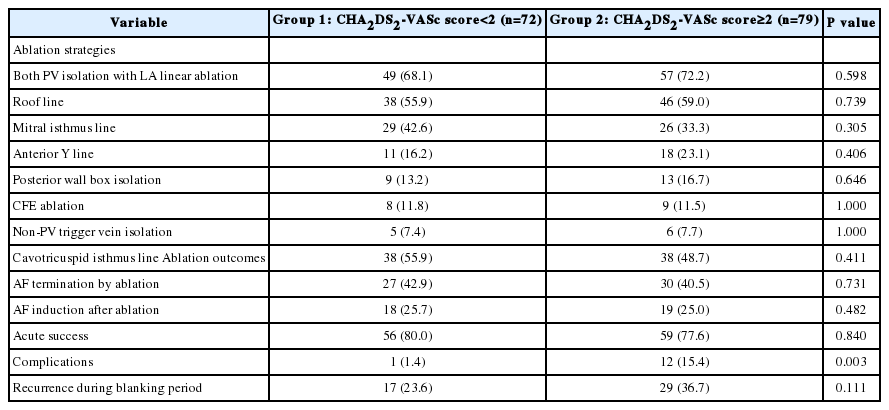
Ablation strategies and outcomes
The ablation strategies and clinical outcomes by CHA2 DS2-VASc score are demonstrated in Table 2. All patients underwent PV isolation and the ablation strategies did not differ between the two groups. However, procedure-related complications were more frequently observed in group 2. Cardiac tamponade occurred in one patient in group 1 and in three patients in group 2. One patient in group 2 had left inferior PV stenosis. Other noted complications noted were subcutaneous hematomas at the vascular access sites. The life-table curve of the recurrence-free survival rate by CHA2 DS2-VASc score is shown in Figure 1. During the mean follow-up period (416.9±101.3 days), AF recurrence after the blanking period was observed more frequently in group 2 (31.6% vs. 18.1%, p=0.046).
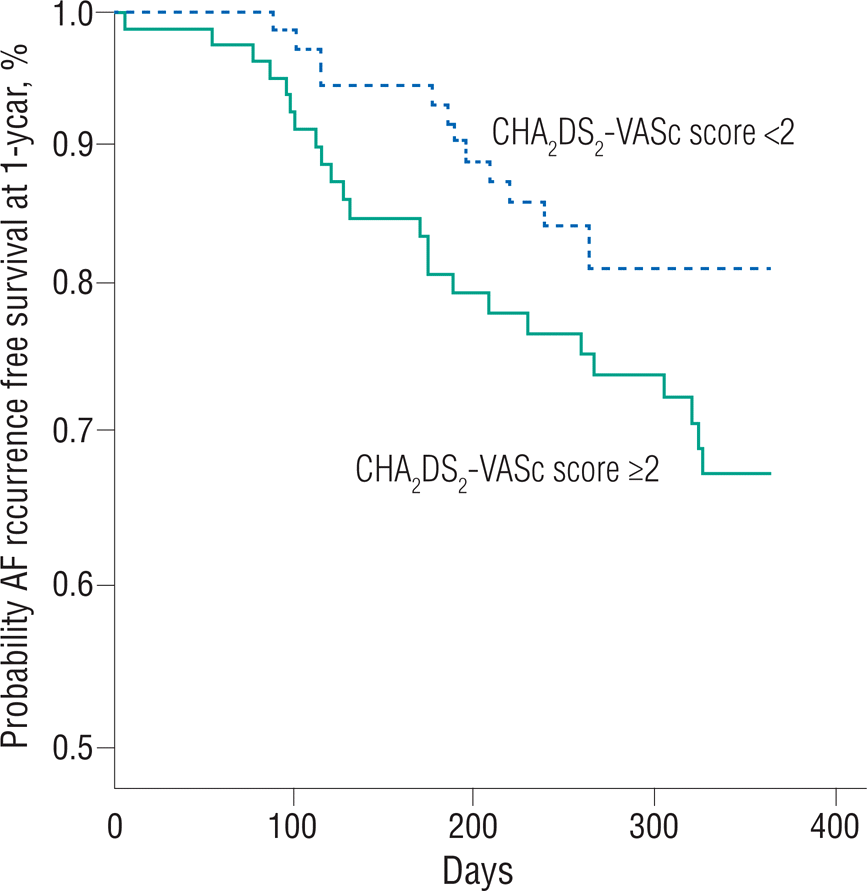
Life-table estimates of the AF recurrence-free survival rate 1 year after the blanking period using CHA2 DS2-VASc score. During the mean fol-low-up period (416.9±101.3 days), AF recurrence after the blanking period is observed more frequently in group 2 (31.6% vs. 18.1%, p=0.046).
AF, atrial fibrillation.
Results of the voltage mapping of the left atrium
The results of the LA voltage mapping during sinus rhythm by CHA2 DS2-VASc score are shown in Figure 2. The mean number of electrograms obtained from the entire LA was 396.9±14.6 and 459.8±208.4 in group 1 and group 2, respectively (p=0.183). The mean LVZ area was 75.64±24.81 cm2 and 94.44±28.09 cm2 in groups 1 and 2, respectively (p=0.005). The relative LVZ area to the mean LA area also differed between the two groups (36.15±12.77% in group 1 vs. 43.03±11.47% in group 2, p=0.026). As shown in Figure 2C, the mean voltage of the LA in group 2 was 0.99±0.31 mV, significantly lower than that in group 1 (1.49±0.67 mV; p=0.001). The mean voltage of the non-LVZs in group 2 was 1.59±0.40 mV, significantly lower than that in group 1 (2.25±0.77 mV; p<0.001). These variables were clearly correlated with hs-CRP level (Figure 3). In the linear regression analysis, the mean LVZ area and LVZ/LA area ratio had a positive linear relationship with hs-CRP level. On the contrary, mean LA voltage was negatively related to hs-CRP.

LA voltage mapping results using the CHA2 DS2-VASc score. The LVZ area (A) and relative LVZ area (B) are significantly higher in group 2. (C) The mean PPV of the LA differs significantly between the two groups.
§ p>0.05.
LA, left atrium; LVZ, low-voltage zone; PPV, peak-to-peak voltage.

Correlations between initial hs-CRP and mean LVZ area (A), LVZ area/LA area ratio (B), mean PPV of the LA (C), and mean non-LVZ (D) are represented by scatter plots and summarized by linear splines. The mean LVZ area and LVZ area/LA area ratio show a positive linear relationship with hs-CRP level. In contrast, the mean LA voltage is negatively correlated to hs-CRP.
hs-CRP, high-sensitivity C-reactive protein; LA, left atrium; LVZ, low-voltage zone; PPV, peak-to-peak voltage.
Prognostic impact of the CHA2 DS2-VASc score on the rhythm outcome
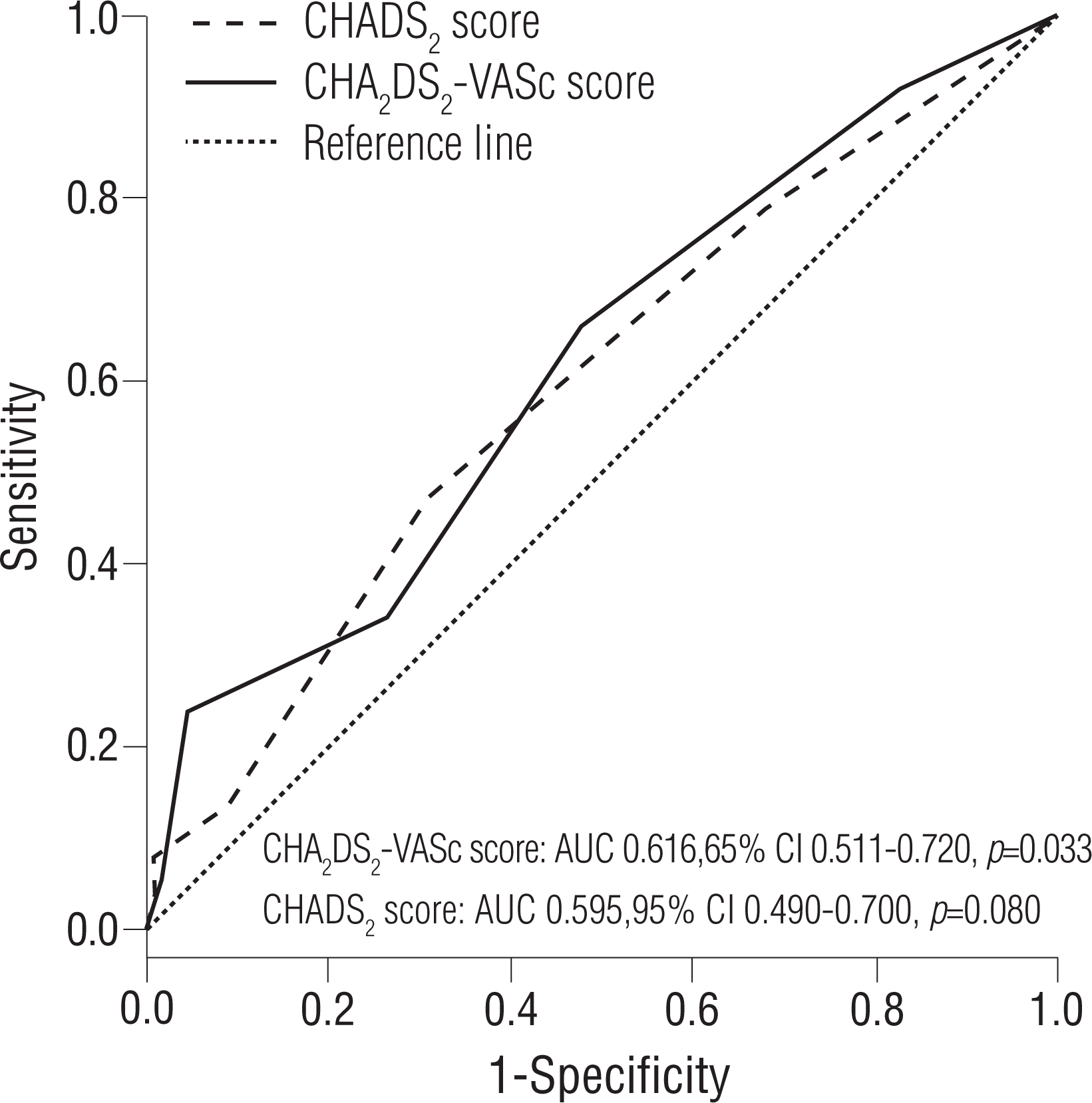
The prognostic impact of the CHA2 DS2-VASc score as a continuous and categorical variable for AF recurrence at 1 year is shown in Table 3. The continuous value of the CHA2 DS2-VASc score was related to post-ablation recurrence in the multivariate Cox regression model. This finding was persistent for the categorical value of the CHA2 DS2-VASc score. When modeled as multivariate continuous variables, a linear and curvilinear relation was seen between the HR for the recurrence-free survival and the CHA2 DS2-VASc score (Figure 4). Other potent predictors for post-ablation recurrence, including mean LVZ area, are also presented in Table 3. The area under the ROC curve showed a modest predictive value of the CHA2 DS2-VASc score for post-ablation recurrence (Figure 5).
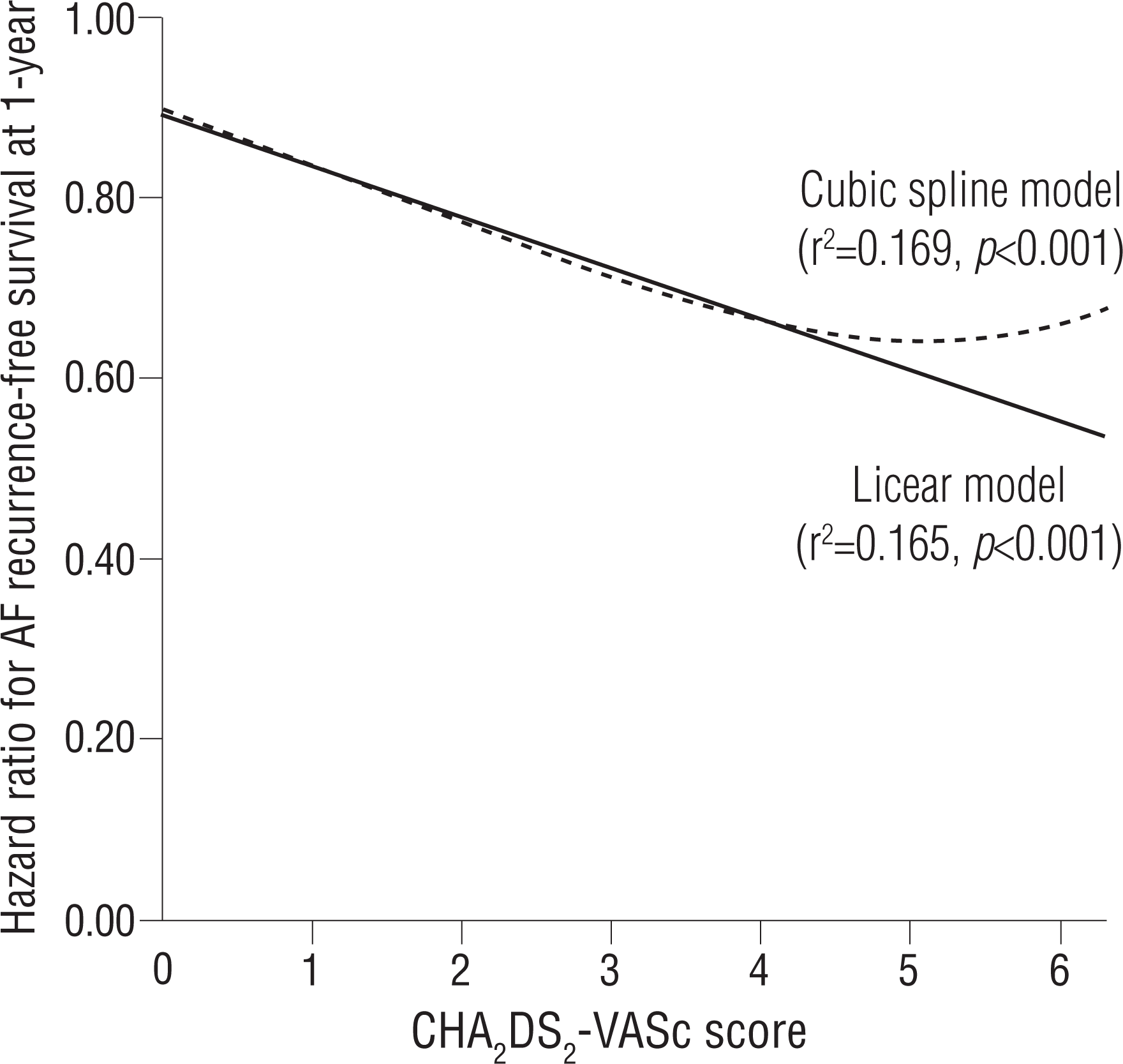
Hazard ratio for AF recurrence-free survival rate 1 year after the blanking period using CHA2 DS2-VASc score (p=0.001). Estimated hazard ratio (in the linear model) = 0.891 − 0.056× CHA2 DS2-VASc score.
AF, atrial fibrillation.
Discussion
Main findings
The present study of 151 patients undergoing radiofrequency ablation of non-valvular AF showed that the overall AF recurrence rate at 1-year of follow-up was 25.2%. The CHA2 DS2-VASc score was an independent predictor of post-ablation recurrence with modest predictive values. This finding was demonstrated as a linear and curvilinear correlation between the continuous value of the CHA2 DS2-VASc score and relative risk of post-ablation recurrence. We also demonstrated that patients with a CHA2 DS2-VASc score ≥ 2 had a lower mean LA voltage and wider mean LVZ area than those with a CHA2 DS2-VASc score <2.
The correlation between the CHA2 DS2-VASc score and post-ablation recurrence
Our observations suggest that the CHA2 DS2-VASc score could be useful for predicting worse outcomes after the catheter ablation of AF. Several explanations seem possible. The present study demonstrated that CHA2 DS2-VASc score was associated with structural remodeling of the LA, which played an important role in AF occurrence and maintenance. Although not investigated in our study, evidence exists of the fact that this structural change in the LA can be commonly accompanied by electrical remodeling, such as changes in the atrial effective refractoriness and conduction velocity.6,7 Atrial remodeling is an independent predictor of AF recurrence after catheter ablation.4,8 Our demonstration of the correlation between the LVZ zone and post-ablation recurrence aligns with those results. The other possible explanation is systemic inflammation, which can affect outcomes after the catheter ablation of AF. In previous studies, an elevated hs-CRP level, a systemic inflammatory biomarker, was related to a high incidence of AF recurrence after cardioversion or catheter ablation of AF.8,9 Furthermore, Chao et al. hypothesized that a high CHADS2 score based on a patient's clinical characteristics may be linked to systemic inflammation.6 Their observation is similar to that of the current study, which showed an elevated hs-CRP level in patients with a high CHA2 DS2-VASc score. In this study, the prevalence of decreased renal function was high in patients with a high CHA2 DS2-VASc score. It has also been proven that impaired renal function is a prognostic factor for worse outcomes after catheter ablation of AF.10 Therefore, these findings can help explain the increased recurrence risk seen in patients with a high CHA2 DS2-VASc score in our study population.
We demonstrated the relative risk of recurrence-free survival at each point of the CHA2 DS2-VASc score. We can assume that the probability of post-ablation recurrence may be conveniently stratified by each point of the CHA2 DS2-VASc score. However, Letsas et al. showed that the predictive value of the CHA2 DS2-VASc score was modest.11 This result is consistent with that of the present study. As mentioned in the previous study, there might be a limitation of the CHA2 DS2-VASc score being a stand-alone risk stratification score for post-ablation AF recurrence,11 but the fact that its value as an independent predictor could remain after the adjustment of other powerful confounders of recurrence that appeared to be consistent in the present and previous studies. Therefore, we believe that this multi-parametric scoring system may be a useful scheme to predict the rhythm outcomes in patients who are candidates for catheter ablation of AF.
Electro-anatomical properties according to the CHA2 DS2-VASc score
The present study showed that patients with high CHA2 DS2-VASc scores had a lower LA voltage and larger LVZ area. A voltage reduction was observed even in the non-LVZ areas in those patients with a high score. Reduced LA voltage is considered an indicator of atrial structural remodeling.12 The occurrence of atrial fibrosis has been emphasized in several experimental and clinical studies evaluating atrial remodeling.7 Cardiac tissue fibrosis including that of the atrium is a reflection of the common result of aging and several heart diseases and systemic diseases including ischemic heart disease, heart failure, hypertension, and DM.13–15 Inflammation, whether systemic or localized in the heart, is a major contributor to cardiac tissue fibrosis.7,14 In fact, several systemic and cardiac diseases that are linked to CHA2 DS2-VASc score can be associated with high inflammatory serum biomarker levels.9,14 The present study also showed that an increased serum hs-CRP level was observed more frequently in patients with a high CHA2 DS2-VASc score and that it was obviously correlated with mean LVZ area and mean LA voltage.
All of this evidence reflects the correlation between the CHA2 DS2-VASc score and LA remodeling noted in our study. Therefore, we hypothesize that our observations can be associated with inflammation degree, which is based on the systemic diseases reflected by the CHA2 DS2-VASc score.
Limitations
There are several limitations to the present study. First, the results must be generalized with caution because our study involved a small sample size with only a single-center experience. However, the number of patients enrolled in our study seems comparable to those of other previous studies investigating the correlation between CHADS2 or CHA2 DS2-VASc scores and outcomes after catheter ablation of AF. We also circumferentially isolated both PV in all patients and performed a stepwise ablation of the substrate modification according to our protocol that complied with the ablation strategy in the current guidelines.1,4 Second, our study population consisted of heterogeneous AF classification. Although the persistence of AF plays an important role in post-ablation prognosis, our study focused on the role of a multi-parametric scoring system such as that used to predict stroke occurrences. Third, we performed Holter monitoring, surface ECG, and event recordings, but the true recurrence rate may be underestimated because of the possibility of asymptomatic episodes of any atrial tachyarrhythmia. Another limitation was that the LA voltage mapping data were available only in 80.8% of our study population. Although this limitation can cause bias on multivariate analysis, we believe that posterior wall box ablation creates an overly large iatrogenic scar compared to other ablation strategies.
Conclusion
Since AF is the result of a complex interplay among several pathophysiological processes, clinical comorbidities, and AF itself,14 a sole predictor may not determine prognosis after catheter ablation. The CHA2 DS2-VASc score appeared to represent atrial remodeling. Moreover, our study showed that the CHA2 DS2-VASc score could be useful in stratifying AF recurrence after a single catheter ablation procedure in patients with non-valvular AF.