Introduction
Atrial fibrillation (AF) is a common and serious arrhythmia that is frequently associated with the development of stroke and systemic embolism. The efficacy of warfarin therapy for the prevention of stroke or systemic embolization has been previously established. Current guidelines recommend maintaining the optimal international normalized ratio (INR) intensity range at between 2.0 to 3.0 in patients with non-valvular AF (NVAF) who are at a moderate to high risk of stroke [1,2]. However, an INR in the therapeutic range (2.0–3.0) occurs in only 50% to 80% of cases even in well-monitored clinical trials [3]. Moreover, optimal intensity of the INR was often lower in the observational or registry data, including the Korean AF registry, where the INR was in the therapeutic range of 42.4% of the time [4]. Among the numerous causes for lower INR intensity, bleeding complications, including intracranial hemorrhage, are the main obstacles in warfarin therapy. Japanese guidelines recommend INR control between 1.6 and 2.6 in patients who are ≥70 years old because old age is associated with the occurrence of severe bleeding events [5]. In addition, hemorrhagic stroke occurs more frequently in Asian patients than in Caucasians [5]. However, the efficacy and safety of low-intensity warfarin therapy in elderly Korean patients with NVAF is unknown. Therefore, we investigated the optimal INR target range and the risks of stroke, systemic embolism, and major bleeding based on age in Korean patients with NVAF.
Subjects and Methods
We enrolled 528 patients with NVAF who have been taking warfarin at our institution between January 2010 and July 2013. Major events were defined as ischemic stroke, systemic embolism, and major bleeding events. Major bleeding was defined based on the International Society for Thrombosis and Hemostasis criteria as clinically overt bleeding accompanied by a decrease in the hemoglobin level of at least 2 g/dL or transfusion of at least 2 units of packed red cells, occurring at a critical site or resulting in death [6]. Patients underwent warfarin therapy for ≥2 years, except for those who developed major events during the follow-up period. The enrolled patients were evaluated monthly by physicians for the occurrence of ischemic stroke, systemic embolism, and major bleeding events, as well as for INR monitoring. The target INR intensity was decided by the physician-in-charge. Knowing the length of the target INR range during the oral anticoagulation period was difficult. We calculated time in therapeutic range (TTR) by the Rosendaal method. Rosendaal et al. [7] proposed a method to determine the time interval between two INR measurements based on the assumption that the actual difference in INR between any two consecutive measurements was linear, and the data were interpolated accordingly. The patients were divided into two groups based on their age. We compared TTR between target INR of 2.0–3.0 and 1.6–2.6 in patients ≥70 years old. Values of INR on the events were counted as INR associated with the events. The INRs that corresponded with the development of ischemic or bleeding events were assessed.
Data were expressed as the mean±SD for continuous variables and percentages for categorical variables. Left ventricular ejection fraction (LVEF), E/E′ and left atrium diameter were assessed by using two-dimensional echocardiography. All comparisons between baseline variables were assessed using the Student t-test for continuous variables and the Pearson chi-square test for categorical variables. For all analyses, a two-sided p value of <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS software (version 18.0 for Windows, SPSS Inc., Chicago, IL, USA)
Results
The baseline characteristics of the study subjects are shown in Table 1. The mean age of the surveyed population was 67±9 years, and 68.4% were male. The mean follow-up period was 1035±255 days. During the study period, 57 patients developed ischemic stroke, systemic embolism, or major bleeding events. Ischemic stroke and systemic embolism occurred in 20 patients with INR between 1.00 and 2.44 (16 ischemic strokes and 4 systemic embolisms). Major bleeding was noted in 37 patients with an INR between 1.74 and no coagulation (exceed laboratory detection capability, more than 10 [7 intracranial hemorrhages, 21 gastrointestinal bleedings, and 9 others]). The TTR during the follow-up period was 45.2±19.2%. No difference was found in the TTR value between patients with and without the events. Incidence rates of ischemic or hemorrhagic events at an INR of <2, 2–3, and >3 were 3.0%, 1.4%, and 20.1% per year, respectively. In patients with ischemic or hemorrhagic events, the age, and CHA2DS2VASc and HAS-BLED scores, were significantly higher compared with those in patients without events.
Table 1.
Baseline clinical characteristics of the patients with and without any events
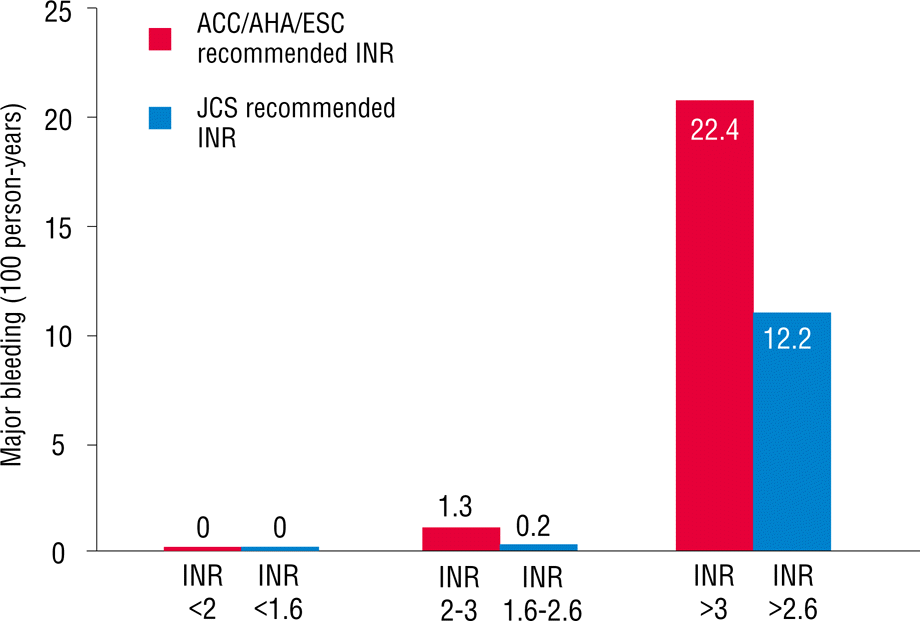
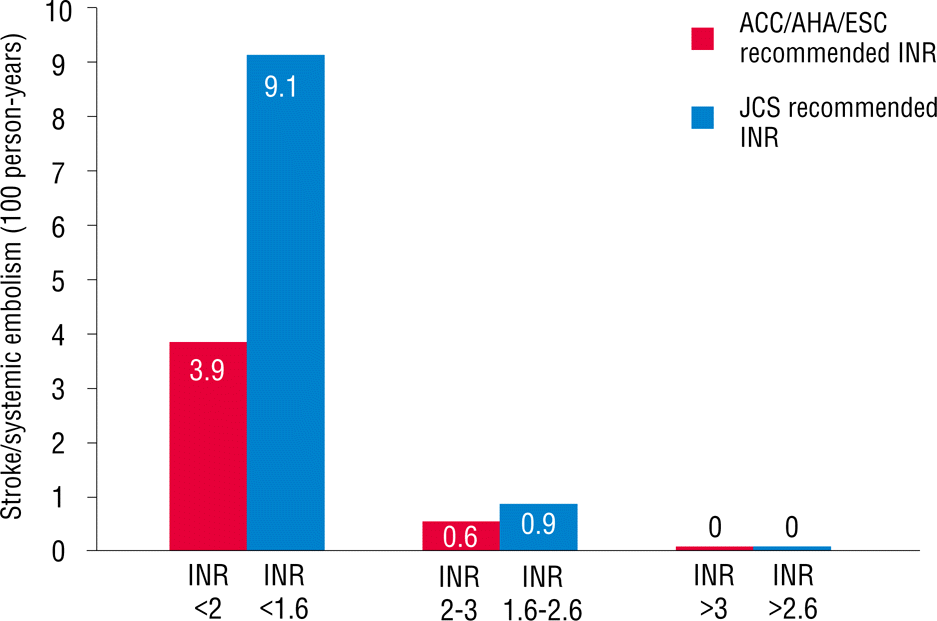
As mentioned earlier, patients were divided into two groups based on age. Overall, 233 patients (44%) were ≥70 years old. More percentage of females were found in this age range, and the body mass index was significantly lower. A history of diabetes mellitus, cerebrovascular accident, and peripheral artery disease in patients who were ≥70 years old was more commonly reported than that in those who were <70 years old. Echocardiographic findings showed that patients who were ≥70 years old had slightly higher ejection fractions and higher E/E′ values compared with those in patients who were <70 years old. No difference was found in the left atrium size between the two groups. In patients who were ≥70 years old, CHADS2, CHA2DS2VASc, and HAS-BLED scores were significantly higher compared with those in patients who were <70 years old. The TTR in patients ≥70 years old was 43.8±19.0% (Table 2). However, no difference was found in the TTR value between patients with and without the events in patients who were ≥70 years old (41.5±19.6% versus 44.1±18.9%, p=0.492). When we applied the INR between 1.6 and 2.6, as recommended by the Japanese AF guidelines for patients who were ≥70 years old, TTR increased from 43.8% to 58.6% (Figure 1). Stroke and systemic embolism event rates within this range slightly increased from 0.6% to 0.9% (Figure 2), whereas hemorrhagic event rates decreased from 1.3% to 0.2% (Figure 3). Overall, any event rates within this TTR range decreased from 1.9% to 1.2% (Figure 4).
Table 2.
Clinical characteristics of the patients according to age
Figure 1.
Changes of time in the therapeutic range among patients ≥70 years old.
INR, international normalized ratio

Figure 2.
Stroke and systemic embolism event rates within the time in the therapeutic range in patients ≥70 years old.
INR, international normalized ratio
Discussion
The main finding of this study is that lower intensity warfarin therapy (INR, 1.6–2.6) is favorable in elderly Korean patients with NVAF. In Korea, the prevalence of AF increased as the population ages. AF will become a serious clinical problem in the future because elderly patients survive longer [8]. The increasing incidence and prevalence of AF increases the likelihood of anticoagulation use in the AF population, which usually includes the elderly [9]. Warfarin is effective for the primary and secondary prevention of both arterial and venous thromboembolic disorders [10,11]. However, anticoagulation intensity correlates with bleeding risk in warfarin-anticoagulated patients [12–14]. In addition, the benefits of warfarin are lost because of the higher rates of intracranial hemorrhage. In addition, racial differences may affect the risk of bleeding [15]. The maintenance doses of warfarin for the Japanese and Chinese are approximately 30% and 40% lower than those for Caucasians, respectively [16]. Genetic determinants of warfarin dosing may alter the effect of warfarin [17,18]. In fact, the incidence of intracerebral hemorrhage was reported to be higher in Japan than in Western countries [19]. Older age particularly increases the risk of bleeding in patients who have been treated with warfarin [20]. In addition, warfarin cessation after major bleeding events increases the risk of stroke, confirming that bleeding prevention can help reduce the risk of stroke in patients taking anticoagulants. Therefore, the Japanese Circulation Society has recommended a lower INR (1.6–2.6) for elderly patients due to the risk of stroke and hemorrhage [5]. However, no Korean data exist on the optimal intensity of INR in warfarin therapy for stroke prevention in elderly patients with NVAF. Our study shows that low intensity INR (1.6–2.6) in Korean patients with NVAF who are ≥70 years old results in slightly increased ischemic event rates, but with markedly decreased hemorrhagic event rates within TTR. Any event rates eventually decreased within TTR.
Our study has some limitations that should be considered. The retrospective analysis of the small single-center data is a major limitation of this study. Therefore, our results should only be considered for hypothesis generation. Secondly, the co-administration of antiplatelet agents might influence bleeding events. However, our data showed that no difference was found in the administration of antiplatelet agents between the two groups based on age.
Conclusion
Our Study adds insight to previous literature showing how low-intensity warfarin therapy (INR 1.6–2.6) might be considered in elderly Korean patients with NVAF. Fear of hemorrhage may prompt some physicians to avoid prescribing anticoagulation, particularly in elderly patients [20,21]. However, anticoagulation may prevent stroke or systemic embolism in elderly patients with a lower target INR.