|
|
International Journal of Arrhythmia 2014;15(2): 39-43.
|
 |
| ECG & EP CASE |
A Case of Atrial Tachycardia
Originating from the Ligament
of Marshall with Migration of
the Earliest Atrial Activation |
 
|
|
 |
 |

Introduction
The ligament of Marshall (LOM) is a vestigial
fold of the epicardium that contains fibrous
bands, small blood vessels, and nervous filaments
enveloped in fat.1 The LOM extends from
the coronary sinus (CS) to the orifice of the left
superior PV (LSPV). It is well known that the
LOM is a non-PV focus of atrial fibrillation (AF)
or atrial tachycardia (AT).2-4
In this report, we describe a case of AT with
migration of the earliest atrial activation along
the pathway of LOM that was terminated by radiofrequency
(RF) ablation inferior to LIPV.
Case
A 50-year-old man with a history of AT was
admitted for electrophysiological study (EPS) and
RF ablation. He was diagnosed with bladder cancer
2 years prior to the admission. Three months
earlier, he began to experience palpitation. Supraventricular
tachycardia was diagnosed and
mild left ventricular (LV) dysfunction (ejection
fraction [EF]=45%) was noted. Rate control for
supraventricular tachycardia was not effective.
During the EPS, the index arrhythmia was an
AT with variable tachycardia cycle length (440-520 ms; Figure 1A). The earliest activation of AT
was localized at the distal CS during tachycardia
(Figure 1B).
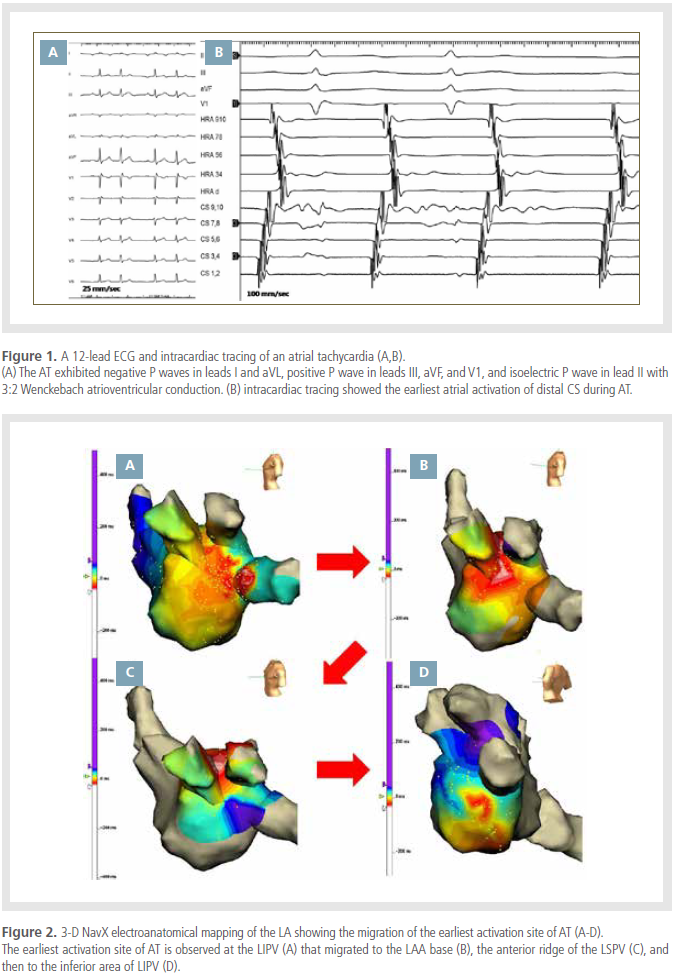
A three-dimensional (3D) electroanatomic
mapping system (NavX; St. Jude Medical, Inc,
St. Paul, MN) was used for activation mapping
of the left atrium during tachycardia. Activation
mapping revealed focal AT arising from the anterior
wall of the LIPV (Figure 2A). However, the
earliest site of AT migrated to the ostium of the
LIPV and the anterior ridge of the LSPV during
mapping. RF ablation was performed targeting
the earliest atrial activation at the anterior wall
of the LIPV. During ablation, sudden PR interval
prolongation and atrioventricular block developed.
AT was terminated during ablation, but
recurred soon afterwards. A second activation
mapping revealed the earliest atrial activation
at the anterior ridge of the left atrial appendage
(LAA) base (Figure 2B). AT slowed during ablation,
but was not terminated. A third activation
mapping showed the earliest atrial activation at
the anterior ridge of the LSPV (Figure 2C). Endocardial
ablation was performed that targeted the
earliest atrial activation site of the anterior ridge
of the LSPV. Epicardial ablation was performed
at the earliest atrial activation site of the vein of
Marshall (VOM) inside the CS. Because of continued
tachycardia after several attempts at RF
and migration of the earliest atrial activation site,
empirical isolation of the antral circumference
of the LSPV and LIPV was attempted. However,
AT was sustained. A last activation mapping revealed
that the earliest atrial activation site of the
AT moved again to a region inferoanterior to the
LIPV (Figure 2D). RF ablation was performed at
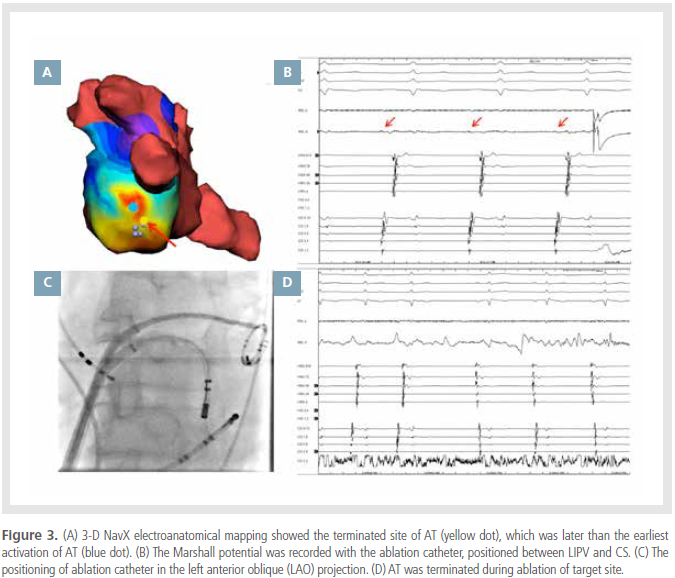
the earliest activation site (Figure 3A, blue dot),
but was not effective. However, discrete electrical
activity was observed inferior to the earliest
atrial activation site of AT, which was considered a Marshall potential (Figure 3B, red arrow). We
decided to ablate at this site, which is a suspected
location of the LOM (Figure 3A yellow dot & 3C).
AT was terminated by RF application at a site
where activation occurred later than at the site of
earliest activation of AT (Figure 3D). No AT was
induced after RF ablation. Follow-up echocardiography
showed improved LV function (EF=52%).
The patient has been well with no recurrence of
any tachyarrhythmias during the 1-year followup
period.
Discussion
In this case, we reported an AT with a migrating
earliest activation site considered to be the
LOM.
The 3D activation map showed that the earliest
activation site of AT migrated from the anterior
wall of the LIPV to the base of the LAA
and then to the anterior ridge of the LSPV. The
earliest activation site was later observed inferior
to the LIPV. The morphology of the P wave
was changed subtly according to the migration
of the earliest atrial activation site of AT. Surprisingly,
AT was not terminated during ablation
at the earliest atrial activation site, but during
ablation inferior to the earliest activation site. A
preceding discrete potential was observed at the
successful ablation site, which was considered a
Marshall potential. Thus, we decided to perform
RF ablation at that site and terminated AT. These
earliest activation sites were located along the
LOM, which courses from the CS obliquely above
the LAA and lateral to the LSPV.5 The migration
of the earliest activation site of AT could be
explained by the complexity of the LOM, which
has multiple myocardial insertions at the LA free
wall and CS, forming a substrate for reentry.1 In this case, several insertion tracts into the left arterial
(LA) free wall might be present, and thus
the earliest activation site of AT could migrate to
another site after RF ablation.
The cycle length of AT was prolonged during
ablation at each earliest activation site of AT, and
sometimes AT was terminated but recurred after
ablation. This phenomenon also supports the
contention that AT originated from the LOM.
The successful ablation site was located inferoposterior
to the earliest activation site of AT. Endocardial
recording at the successful ablation site
showed double component potentials. Recently,
Kuroki et al. also reported an AT arising from the
LOM with a successful ablation site posteroinferior
to the initial earliest activation site.2 In that
case, a fractionated local electrogram preceding
P wave onset was found at the successful ablation
site. Another report of focal AT originating
from the LOM also showed discrete a electrical
potential (Marshall potential) preceding the atrial
electrogram.4 The difference between the earliest
activation site and the successful ablation site
during AT could be because the earliest activation
site in the activation map might be the exit
site from the LA during AT arising from LOM,
however, the successful ablation site with double
potential might be the area of insertion of the
Marshall bundle into the LA endocardial wall. In
our case, local fractionated potentials were observed at the successful ablation site, albeit later
than the earliest atrial activation of AT. The initial
potential was regarded as a Marshall potential,
whereas the second potential might be atrial
activation (Figure 3B).
In this case, we failed to terminate AT during
epicardial ablation inside the CS, but succeeded in
terminating AT during endocardial ablation. Previously,
Hwang et al. reported successful ablation
of AF arising from the LOM by RF application
from the LA endocardium with the guidance of a
catheter within VOM.6 Subsequently, the endocardial
approach was supported by an anatomical
study showing that the Marshall bundle could
directly insert distally into the posterior atrial free
wall superior to the CS.1
In conclusion, we reported AT originating from
the LOM, which showed migration of the earliest
atrial activation during AT. The successful ablation
site could be different from the earliest activation
site, and local fractionated potentials could
be promising markers for ablation of AT.
References
- Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, Fishbein MC. The ligament of marshall: A structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324-1327.
- Kuroki K, Tada H, Kunugida F, Sekiguchi Y, Machino T, Yamasaki H, Igarashi M, Aonuma K. Hybrid epicardial and endocardial ablation of a persistent atrial tachycardia arising from the marshall bundle: The importance of a detailed analysis of the local potentials. Heart Vessels. 2014.
- Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176-3183.
- Polymeropoulos KP, Rodriguez LM, Timmermans C, Wellens HJ. Images in cardiovascular medicine. Radiofrequency ablation of a focal atrial tachycardia originating from the marshall ligament as a trigger for atrial fibrillation. Circulation. 2002;105:2112-2113.
- Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18-35.
- Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503-1505.
|
|
|
|