|
|
International Journal of Arrhythmia 2014;15(3): 13-23.
|
 |
| ORIGINAL ARTICLES |
Relationship between Genetic Polymorphisms of
Angiotensin-Converting Enzyme and the Degree
of Electroanatomical Remodeling of the Atrium
in Patients with Non-valvular Atrial Fibrillation |
|
 |
 |

Introduction
Atrial fibrillation (AF) is the most common clinical
arrhythmia. It is associated with cardiovascular
morbidity and is related to increased disability.1,2 The pathophysiology of AF is heterogeneous,3 and
long-standing AF is associated with changes in left
atrial (LA) morphology. AF alters the
electrophysiological properties of atrial myocytes and
causes alterations in the structure of the atrial
myocardium.4,5 The longer the duration of AF, the
more persistent it becomes due to atrial remodeling.
Both electrical remodeling and structural remodeling
beget AF, and an increase in AF burden leads to
more vulnerable substrates.6 The structural
remodeling is related to interstitial fibrosis,
downregulation of gap junctions, and enlargement of
atrial chamber size (critical mass).7,8 The degree of
structural remodeling as measured by LA size affects the clinical outcome of rhythm control strategies in
patients with AF.9 LA scarring is also an
independent predictor of procedure failure after
radiofrequency catheter ablation (RFCA) of AF.10 The
renin-angiotensin system (RAS) is involved in many
cardiovascular diseases, including myocardial fibrosis
and hypertrophy, and AF is associated with
activation of the RAS in the atria.11 Angiotensinconverting
enzyme (ACE) stimulates fibroblast
proliferation, collagen synthesis, and atrial structural
remodeling in patients with AF.12 Ravn et al.13
reported that the angiotensinogen (AGT) A-20C
genotype in combination with the ACE I/D genotype
predicts an increased risk of AF, but few studies have searched for a genetic predisposition to LA structural remodeling in patients with AF.
Therefore, we hypothesized that ACE
polymorphisms are associated with the degree of
atrial structural remodeling in patients with AF. We
investigated the relationship between ACE
polymorphism and LA volume measured by a
3D-spiral computed tomography (CT) scan or LA
voltage calculated by 3D-electroanatomical mapping
in Korean AF patients who underwent RFCA.

Methods
Patient Selection
The study protocol was approved by the Institutional
Review Board of our institute. All patients provided
written informed consent. The study enrolled 351
patients with AF (male:female=282:69, mean
age=54.2±11.1 years) who underwent RFCA. Among
them, 235 patients had paroxysmal AF (PAF), and 116
had persistent AF (PeAF). The exclusion criteria were
as follows: (1) permanent AF refractory to electrical cardioversion; (2) LA sizes >50 mm measured by
echocardiogram; (3) AF with rheumatic valvular
disease; (4) associated structural heart disease; (5)
prior AF ablation; and (6) sinus rhythm not maintained
for LA voltage mapping before RFCA. Patients with
the presence of an LA thrombus were excluded by
transesophageal echocardiography. We imaged all
patients with a 3D-spiral CT (64 Channel, Light
Speed Volume CT, Philips, Brilliance 63, Amsterdam,
Netherlands) to visually characterize the anatomy of
the LA and LVs. Transthoracic echocardiography was
performed in all patients and the anterior-posterior
(AP) diameter of the LA, left ventricular ejection
fraction (LVEF), LV diastolic function measured by
E/E' , LV end-systolic dimension (LVESD), and LV
diastolic dimension (LVEDD) were measured.
Electrophysiological Mapping
Intracardiac electrograms were recorded using a
Prucka CardioLabTM Electrophysiology system
(General Electric Health Care System Inc., Milwaukee, WI, USA). For AF RFCA (n=351), we
used 5 mapping catheters and a deflectable 3.5-mm,
7 Fr open irrigation tip ablation catheter (Celsius,
Johnson & Johnson Inc., Diamond Bar, CA, USA).
The catheter ablation procedures were performed
using 3D electroanatomical mapping (NavX system,
St. Jude Medical Inc., Minneapolis, MN, USA) in all
patients. Before the catheter ablation, we generated
an LA 3D electroanatomical map and voltage map by
obtaining contact bipolar electrograms from
approximately 100-150 points throughout the LA
endocardium of the high right atrium with pacing
cycle lengths of 500 ms. The bipolar electrograms
were filtered between 32-300 Hz. Color-coded
voltage maps were generated by recording bipolar
electrograms and measuring the peak-to-peak
voltage.
Analyses of LA Remodeling: 3D-Spiral CT and Electroanatomical Voltage Map
The 3D-spiral CT images of the LA were analyzed
on an imaging processing workstation (Aquarius,
Terarecon, Inc, Concord, MA, USA). The curvilinear
lengths of the LA were measured at the following
linear ablation sites: the bilateral antral ablation line,
roof line, posterior inferior line, left lateral isthmus
line, and anterior line. Each LA image was divided
into the following parts according to embryological
origin: the venous LA (posterior LA including the
antrum and posterior wall), LA appendage (LAA),
and anterior LA (excluding the LAA and venous
LA).14 We also measured the curvilinear lengths of
circumferential pulmonary vein ablation, the roof
line, posterior inferior line, anterior linear line, and
left lateral isthmus line as described in a previous
study.15
We analyzed the color-coded LA electroanatomical voltage maps in the AP and posterior-anterior (PA)
views. The low voltage areas ≤0.2 mV were coded
with a gray area and the high voltage areas >5.0
mV were colored purple. The reference distance was
measured by the inter-electrode distances of
coronary sinus catheters (Duodecapolar Catheter, St.
Jude Medical Inc. Minnetonka, MN, USA). The LA
was divided into 4 quadrants in each of the views.
To quantify the mean voltage of the LA, the percent
area represented by each color was calculated using
customized software (Image-Pro) with reference to a
color scale bar.15
Genetic Polymorphism Analyses
We selected haplotype-tagging single nucleotide
polymorphisms (SNPs) of the ACE gene using the
HapMap Japanese (JPT) data bank (http://www.hapmap.org) and NCBI SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). To identify eligible
tag SNPs in our population, we carried out a pilot
study by genotyping for 16 selected SNPs in 48
Korean subjects. We identified 7 candidate SNPs
(C-3927T, A-262T, P405P, T776A, ACE I/D,
F1129F, and C2359). Genomic DNA was extracted
from whole blood samples using a commercially
available kit (Qiagen, Valencia, CA, USA).
Genotyping for 6 of the SNPs was conducted by a
single-base extension method using the SNaPShotTM
Assay kit (Applied Biosystems, Foster City, CA,
USA), and genotyping of the I/D polymorphism was
performed with polymerase chain reaction as
described previously.16
Data Analyses
We selected ACE variants that were related to the
degree of structural remodeling, as indicated by the entire LA volume, regional LA volume, regional
curvilinear LA lengths, mean and regional LA
voltage, and the LA/LVEDD ratio measured by
echocardiography.

Statistical analyses were
performed using the SPSS statistical package release
17.0.1 (SPSS, Inc., Chicago, IL, USA). Data were
expressed as means ± standard deviations (SDs).
Between-group data for baseline characteristics
were compared with the Student’s unpaired t-test
for continuous data and the χ2 test for categorical
data. For statistical analyses, we defined the cutoff
as the median rounded to 0.1 decimal places and
validated it by a receiver-operating characteristic
(ROC) curve analysis. All genotype frequencies were
in Hardy-Weinberg equilibrium (HWE) (p>0.05).
HWE of the genotype frequencies was evaluated
using a χ2 test. In single-locus analyses, we first
compared the allele and genotype frequencies
between the cases and controls with the χ2 test or
Fisher’s exact test. Statistical significance was
defined as p<0.05.
Results
ACE Variants Associated with LA Structural Remodeling in Patients with AF
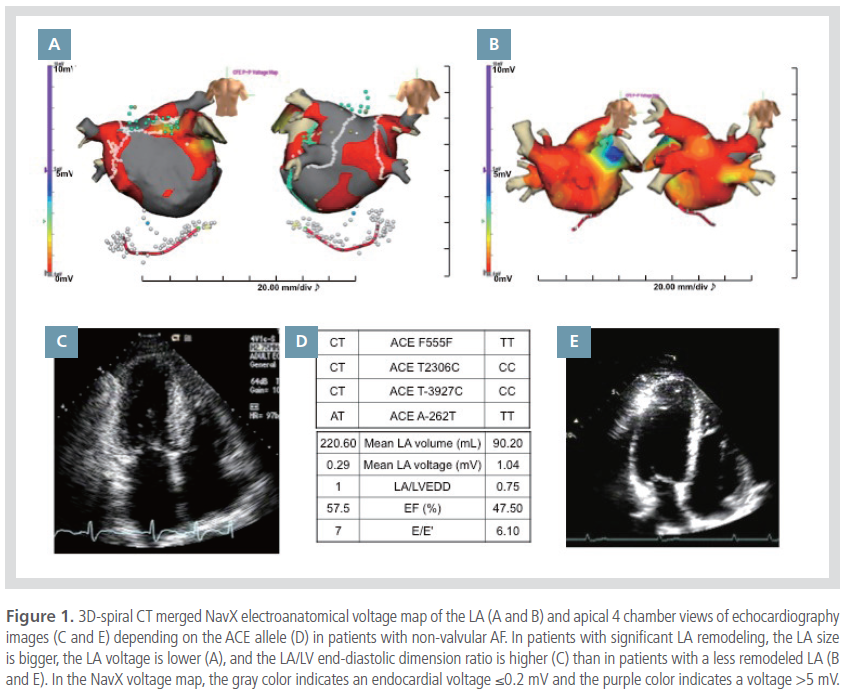
Figure 1 shows representative examples of highly
remodeled (Figures 1A and 1C) and less remodeled
LAs (Figures 1B and 1E) in patients with AF, and
their ACE genotypes (Figure 1D). The patients with
significant electroanatomical remodeling of the LA
show an enlarged LA volume, a low endocardial
voltage (Figure 1A), and a high LA/LVEDD ratio
(Figure 1C). In contrast, the patients with a less
remodeled LA had a relatively small LA volume with
a high endocardial voltage (Figure 1B) and a low LA/
LVEDD ratio (Figure 1E).

Among the 7 SNPs evaluated, 5 polymorphisms of
the ACE gene were associated with structural
remodeling of LA in the 351 patients with nonfamilial
non-valvular AF. Table 1 summarizes the
relationships between the ACE variants and the
degree of structural remodeling of the LA. The
F1129F C (p=0.024), P405P T (p=0.030), T-3927C T
(p=0.030), and A-262T A (p=0.027) ACE alleles
were associated with an enlargement of LA volume.
LA enlargement relative to LV size (LA/LVEDD)
measured by echocardiography was significantly
higher in patients with the ACE F1129F C allele
(p=0.039) and the P405P T allele (p=0.026) than in
other patients. The ACE F1129F T allele (p=0.021)
and ACE D carriers (DD+ID) allele (p=0.021) were
the predominant genotypes in patients with low
mean LA voltage.
ACE Variants Related to LA Structural Remodeling
Measured by LA Volume
Table 2 summarizes the segmental volume and
segmental curvilinear length of the LA adjusted for
body surface area (BSA) with respect to the F1129F
genotype. We also compared the mean and regional
LA voltage and echocardiography parameters.
Generally, patients with the F1129F C allele had
larger total and regional LA volumes (p<0.01) and
longer regional curvilinear lengths of the LA
(p<0.05) than those with the F1129F TT genotype.
In contrast, patients with the F1129F T allele had a
lower LA voltage than those with the F1129F CC
genotype (p<0.05). The characteristics of LA
remodeling in patients with the ACE P405P allele,
T-3927 T allele, and A-262T allele are listed in
Table 3.
ACE Variants and Clinical Outcomes after
Catheter Ablation of AF
The clinical recurrence rate of AF after a 3-month
blanking period was 20.45% during the 28.29±5.83
month follow-up. We did not find ACE-related
polymorphisms associated with long-term clinical
recurrence after catheter ablation. However, the
ACE F1129F T allele, which was related to a low
endocardial LA voltage, was associated with a higher
early recurrence rate (within 3 months) (44.9%) after
RFCA than the ACE F1129F CC genotype (27.5%,
p=0.0217).
Discussion
This study demonstrated the association between
ACE polymorphisms and structural remodeling of
the LA measured by LA volume and endocardial
voltage. ACE polymorphism also affected early
recurrence after catheter ablation of AF. A genetic
predisposition of specific ACE genotypes predicts
atrial remodeling and may provide the basis for a
treatment strategy.
The Mechanisms of Electroanatomical Remodeling
of AF
AF begets AF. Wijffels et al.6 reported that the
higher the AF burden, the more persistent it
becomes owing to atrial remodeling. There are two
kinds of atrial remodeling. Electrical remodeling is a
process of ion channel adaptation to tachyarrhythmia,
4,6 and structural remodeling is the
change in LA volume, voltage, and conduction
velocity by matrix remodeling.15,17 The former is
reversible by maintaining a sinus rhythm, while the
latter is irreversible.18 Because structural remodeling changes the morphology and endocardial voltage of
the atrium, clinicians call it electroanatomical
remodeling. Electroanatomical remodeling is
provoked by mechanical stretch-related extracellular
matrix genes.19,20 Profibrotic signals including
angiotensin II,21 TGF-β,22 platelet-derived growth
factor (PDGF),23 or connective tissue growth factor
(CTGF)24 are known to proceed extracellular matrix
remodeling. Those profibrotic signals also induce the
proliferation of myofibroblasts.25 Myofibroblasts
contribute to collagen deposition with apoptosis or
necrosis of cardiomyocytes,20 electroanatomical
remodeling,15,17 and the non-reentrant mechanism of
AF by automaticity.20,26 Recently, we reported a
higher LA volume, slower conduction velocity, lower
endocardial voltage, and poorer clinical outcome
after catheter ablation in patients with significant
electroanatomical remodeling than those with a less
remodeled LA.15,17 However, there are individual
differences in the degree and rate of
electroanatomical remodeling of the LA in patients
with AF. Therefore, we determined the ACE
polymorphisms related to angiotensin II, one of the
profibrotic signals, and their association with the
degree of electroanatomical remodeling of AF.
Genetic Polymorphisms of Renin-Angiotensin
System and Matrix Remodeling
The RAS is involved in many cardiovascular
diseases, including heart failure and myocardial
infarction related to oxidative stress, inflammation,
or mechanical overload.27,28 The ACE D allele
(DD+ID) is more common in patients with significant
LV remodeling after myocardial infarction.29,30 The
ACE DD genotype and the AT1R A1166C (AC+CC)
genotype are associated with the LV mass index and
diastolic heart failure.31 However, genetic studies of
the RAS related to AF or atrial remodeling are
limited. Recently, Tsai et al.32 reported that the ACE
I/D polymorphism and several variants in the
angiotensinogen and angiotensin II type I receptor
are associated with nonfamilial structural AF.
Watanabe et al.33 reported that the ACE D allele is
associated with a longer PR interval in patients with
lone AF. In this study, we reported several ACE
polymorphisms associated with electroanatomical
remodeling of the LA in patients with AF.
Specifically, it was associated with LA enlargement,
reduced endocardial voltage, and early recurrence
after catheter ablation. These ACE polymorphisms
might be useful for the detection of patients with AF
who are susceptible to structural remodeling.
Although we found an association of these genes
with LA remodeling, they were not significantly
associated with LV size or LV systolic and diastolic
function in this highly selected and relatively
homogeneous patient group with non-valvular AF.
Clinical Implications
ACE polymorphisms associated with
electroanatomical remodeling of the LA might be
useful for the early detection of susceptible patients
and prevention of the progression to chronic
permanent AF with electroanatomical remodeling.
Upstream therapy with an ACE inhibitor or
angiotensin II receptor blocker prevents LA
remodeling and is used as a tailored
management.34,35 Those variants also may justify the
early intervention with catheter ablation and
improve the prognostic value and clinical outcome.
Study Limitations
The patients included in this study were a highly selected group referred for rhythm control, and the
number of patients was limited. The exclusion of
patients with large atria (greater than 50 mm) may
influence the results and clinical outcomes. Because
we acquired voltage maps by point-by-point
contact mapping, they did not reflect a
spatiotemporally homogeneous distribution. We
analyzed 3D voltage maps using 2D measurements.
Conclusion
We demonstrated the association between ACE
polymorphisms and structural remodeling of the LA
as measured by LA volume and endocardial voltage.
Individuals with specific ACE genotypes are
predisposed to atrial remodeling and these genotypes
may provide the foundation for a therapeutic
strategy.
Acknowledgements
This work was supported by grants from the Korea
Health 21 R&D Project, the Ministry of Health and
Welfare (A085136) and the National Research
Foundation of Korea (NRF) funded by the Ministry
of Science, ICT & Future Planning (MSIP; 7-2013-
0362).
Conflicts of Interest
The authors have no conflict of interest disclosures.
References
- van den Berg MP, van Gelder IC, van Veldhuisen DJ. Impact of atrial fibrillation on mortality in patients with chronic heart failure.
Eur J Heart Fail.
2002;4:571-575
- Khairy P, Nattel S. New insights into the mechanisms and management of atrial fibrillation.
CMAJ.
2002;167:1012-1020.
- Nattel S, Opie LH. Controversies in atrial fibrillation.
Lancet.
2006;367:262-272.
- Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms.
Circulation.
1996;94:2968-2974.
- Nattel S. New ideas about atrial fibrillation 50 years on.
Nature.
2002;415:219-226.
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats.
Circulation.
1995;92:1954-1968.
- van der Velden HM, Ausma J, Rook MB, Hellemons AJ, van Veen TA, Allessie MA, Jongsma HJ. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat.
Cardiovasc Res.
2000;46:476-486.
- Zou R, Kneller J, Leon LJ, Nattel S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium.
Am J Physiol Heart Circ Physiol.
2005;289:H1002-1012.
- Shin SH, Park MY, Oh WJ, Hong SJ, Pak HN, Song WH, Lim DS, Kim YH, Shim WJ. Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation.
J Am Soc Echocardiogr.
2008;21:697-702.
- Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J, Burkhardt JD, Saliba W, Schweikert RA, Natale A. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction.
Circulation.
2005;112:627-635.
- Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation.
J Am Coll Cardiol.
2000;35:1669-1677.
- Rosenkranz S. Tgf-beta1 and angiotensin networking in cardiac remodeling.
Cardiovasc Res.
2004;63:423-432.
- Ravn LS, Benn M, Nordestgaard BG, Sethi AA, Agerholm-Larsen B, Jensen GB, Tybjaerg-Hansen A. Angiotensinogen and ace gene polymorphisms and risk of atrial fibrillation in the general population.
Pharmacogenet Genomics.
2008;18:525-533.
- Douglas YL, Jongbloed MR, Gittenberger-de Groot AC, Evers D, Dion RA, Voigt P, Bartelings MM, Schalij MJ, Ebels T, DeRuiter MC. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation.
Am J Cardiol.
2006;97:662-670.
- Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, Choi JI, Hwang C, Kim YH. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: Comparison of three-dimensional computed tomographic images and voltage mapping.
J Cardiovasc Electrophysiol.
2009;20:1349-1356.
- Chiang FT, Hsu KL, Chen WM, Tseng CD, Tseng YZ. Determination of angiotensin-converting enzyme gene polymorphisms: Stepdown PCR increases detection of heterozygotes.
Clin Chem.
1998;44:1353-1356.
- Park JH, Pak HN, Kim SK, Jang JK, Choi JI, Lim HE, Hwang C, Kim YH. Electrophysiologic characteristics of complex fractionated atrial electrograms in patients with atrial fibrillation.
J Cardiovasc Electrophysiol.
2009;20:266-272.
- Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure.
Circulation.
2004;109:412-418.
- Riser BL, Cortes P, Heilig C, Grondin J, Ladson-Wofford S, Patterson D, Narins RG. Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells.
Am J Pathol.
1996;148:1915-1923.
- Cardin S, Libby E, Pelletier P, Le Bouter S, Shiroshita-Takeshita A, Le Meur N, Leger J, Demolombe S, Ponton A, Glass L, Nattel S. Contrasting gene expression profiles in two canine models of atrial fibrillation.
Circ Res.
2007;100:425-433.
- Weber KT, Sun Y, Katwa LC, Cleutjens JP. Tissue repair and angiotensin II generated at sites of healing.
Basic Res Cardiol.
1997;92:75-78.
- Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1).
Mol Genet Metab.
2000;71:418-435.
- Ivarsson M, McWhirter A, Borg TK, Rubin K. Type I collagen synthesis in cultured human fibroblasts: Regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers.
Matrix Biol.
1998;16:409-425.
- Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and -independent pathways.
Cardiovasc Res.
2003;60:315-325.
- Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: Involvement in cardiac hypertrophy.
Circ Res.
2002;91:1103-1113.
- Mohabir R, Ferrier GR. Effects of ischemic conditions and reperfusion on depolarization-induced automaticity.
Am J Physiol.
1988;255:H992-999.
- Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension.
Circ Res.
1990;67:1355-1364.
- Hanatani A, Yoshiyama M, Kim S, Omura T, Toda I, Akioka K, Teragaki M, Takeuchi K, Iwao H, Takeda T. Inhibition by angiotensin II type 1 receptor antagonist of cardiac phenotypic modulation after myocardial infarction.
J Mol Cell Cardiol.
1995;27:1905-1914.
- Nagashima J, Musha H, So T, Kunishima T, Nobuoka S, Murayama M. Effect of angiotensin-converting enzyme gene polymorphism on left ventricular remodeling after anteroseptal infarction.
Clin Cardiol.
1999;22:587-590.
- Ohmichi N, Iwai N, Maeda K, Shimoike H, Nakamura Y, Izumi M, Sugimoto Y, Kinoshita M. Genetic basis of left ventricular remodeling after myocardial infarction.
Int J Cardiol.
1996;53:265-272.
- Wu CK, Luo JL, Wu XM, Tsai CT, Lin JW, Hwang JJ, Lin JL, Tseng CD, Chiang FT. A propensity score-based case-control study of renin-angiotensin system gene polymorphisms and diastolic heart failure.
Atherosclerosis.
2009;205:497-502.
- Tsai CT, Hwang JJ, Chiang FT, Wang YC, Tseng CD, Tseng YZ, Lin JL. Renin-angiotensin system gene polymorphisms and atrial fibrillation: A regression approach for the detection of genegene interactions in a large hospitalized population.
Cardiology.
2008;111:1-7.
- Watanabe H, Kaiser DW, Makino S, MacRae CA, Ellinor PT, Wasserman BS, Kannankeril PJ, Donahue BS, Roden DM, Darbar D. Ace i/d polymorphism associated with abnormal atrial and atrioventricular conduction in lone atrial fibrillation and structural heart disease: Implications for electrical remodeling.
Heart Rhythm.
2009;6:1327-1332.
- Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation.
J Am Coll Cardiol.
2003;41:2197-2204.
- Madrid AH, Bueno MG, Rebollo JM, Marin I, Pena G, Bernal E, Rodriguez A, Cano L, Cano JM, Cabeza P, Moro C. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: A prospective and randomized study.
Circulation.
2002;106:331-336.
|
|
|
|